TAXIFOLIN 3-O-RHAMNOSIDE
Synonym(s):Astilbin;Dihydroquercetin 3-rhamnoside;Taxifolin 3-rhamnoside
- CAS NO.:29838-67-3
- Empirical Formula: C21H22O11
- Molecular Weight: 450.4
- MDL number: MFCD20274680
- EINECS: 694-695-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-11 16:58:07
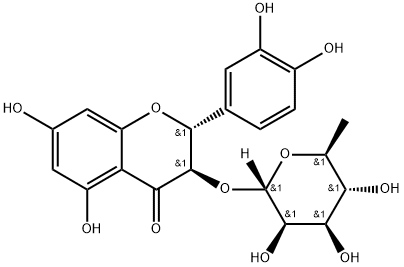
What is TAXIFOLIN 3-O-RHAMNOSIDE?
Description
Astilbin is a flavonoid that has been found in S. glabra and has diverse biological activities. It inhibits cisplatin-induced increases in apoptosis and accumulation of reactive oxygen species (ROS) in HEK293 cells when used at a concentration of 200 μM. Astilbin (50 mg/kg) increases renal glutathione (GSH) levels and superoxide dismutase (SOD) and catalase activity and reduces serum creatinine and blood urea nitrogen (BUN) levels, renal IL-1β, IL-6, and TNF-α levels, apoptosis in kidney tissue, and kidney injury in a mouse model of cisplatin-induced nephrotoxicity. It reduces loss of dopaminergic neurons in the substantia nigra and increases striatal GSH levels and SOD activity in a mouse model of MPTP-induced Parkinson''s disease when administered at a dose of 50 mg/kg per day. Astilbin also reduces descent time in a pole test and increases traction test score in a mouse model of Parkinson''s disease, indicating reduced motor deficits. It reduces cell viability of MDA-MB-231 and MCF-7 cells (IC50s = 167.9 and 191.6 μM, respectively), as well as inhibits migration and increases apoptosis when used at concentrations of 50 and 200 μM. Astilbin (20 mg/kg every other day for 14 days) reduces tumor growth in an MCF-7 mouse xenograft model.
The Uses of TAXIFOLIN 3-O-RHAMNOSIDE
Astilbin displays anti-depressant activity involving monoaminergic neurotransmitters an the BDNF signal pathway. Anti-oxidant.
Definition
ChEBI: A flavanone glycoside that is (+)-taxifolin substituted by a alpha-L-rhamnosyl moiety at position 3 via a glycosidic linkage.
Properties of TAXIFOLIN 3-O-RHAMNOSIDE
| Melting point: | 180 °C (decomp) |
| Boiling point: | 801.1±65.0 °C(Predicted) |
| Density | 1.74 |
| storage temp. | Sealed in dry,2-8°C |
| solubility | H2O: soluble1mg/mL, clear, colorless |
| pka | 7.34±0.60(Predicted) |
| form | A crystalline solid |
| color | White to off-white |
Safety information for TAXIFOLIN 3-O-RHAMNOSIDE
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for TAXIFOLIN 3-O-RHAMNOSIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran
You may like
-
 Astilbin from Engelhardtia roxburghiana 98% (HPLC) CAS 29838-67-3View Details
Astilbin from Engelhardtia roxburghiana 98% (HPLC) CAS 29838-67-3View Details
29838-67-3 -
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3