Tannic acid
Synonym(s):Gallotannin;Tannic acid;Tannin
- CAS NO.:1401-55-4
- Empirical Formula: C76H52O46
- Molecular Weight: 1701.2
- MDL number: MFCD06412477
- EINECS: 215-753-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 18:23:36
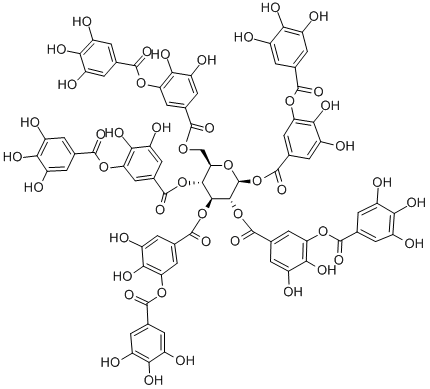
What is Tannic acid?
Absorption
After ingestion it has poor bioavailability, due to large size, high affinity to bound to plasma proteins and low lipid solubility. Its main actions are due to local effects.
Toxicity
LD50 is 2.26 g/Kg in rats.
Description
Tannic acid is a polyphenol found in many herbs and fruits. The pharmacological activities of tannic acid include anticancer, antioxidant, antiproliferative, and anti-inflammatory. It is used in treating diarrhea, ulcers, toothache, wounds, diaper and skin rashes, ingrown toenails, and for stopping bleeding.
Chemical properties
Tannic acid or hydrolysable gallotannin is a complex polyphenolic organic structure that yields gallic acid and either glucose or quinic acid as hydrolysis products. Tannic acid is odorless or has a faint, characteristic odor and an astringent taste.
Occurrence
Reported found in Quercus oliver and related species; Tara (Caesalpinia spinosa); and various sumac species, including Rhus semialata, R. coriaria, R. glabra and R. typhia.
The Uses of Tannic acid
Tannic acid was historically used for the treatment of diarrhea, topically to dress skin burns and rectally for treatment of unspecified rectal disorders. It may also be employed in dyeing, ink manufacturing, paper sizing, food and wine processing, and the production of gallic acid and catechol.
Indications
Tannic acid is indicated for cold sores, fever blisters, diaper rash, minor burn or sunburn and prickly heat. Vaginally, tannic acid is used as a douche for leukorrhea. It has been also indicated for sore throat, inflamed tonsils, spongy or receding gums, and acute dermatitis.
Background
Tannic acid is a polyphenolic compound. It is a type of the commercially available tannins. It acts as a weak acid. Tannic acid is found in the nutgalls formed by insects on twigs of certain oak trees (Quercus infectoria and other Quercus species). It is removed and used as medicine. In the old days it was used as antidote against different poisons. Nowadays, tannic acid is applied topically for the treatment of cold sores, diaper rash, fever blisters and poison ivy. Tannic acid is also taken by mouth and applied directly for bleeding, chronic diarrhea, dysentery, bloody urine, painful joints, persistent coughs, and cancer.
What are the applications of Application
Gallotannin is an inhibitor of NOS3 and a weak inhibitor of NOS2 and NOS1
Definition
tannic acid: A yellowish complexorganic compound present in certainplants. It is used in dyeing as a mordant.
Preparation
Tannic acid is obtained by solvent extraction from the nutgalls or the excrescences that form on the young twigs of Quercus olivier and allied species of Quercus L.; from the seed pods of Tara (Caesalpinia spinosa); or from the nutgalls of various sumac species, including Rhus semialata, R. coriaria, R. glabra and R. typhia.
Composition
Main constituents include three crystalline alkaloids—aspidospermine, quebrachine and quebrachamine (chemically identical to yohimbine)—responsible for the tonic and antispasmodic physiological action. A. quebrachoblanco bark contains 0.3 to 1.5% alkaloids (aspidospermine 30%, quebrachine 10%, deacetylaspidospermine 5%, aspidospermatidine 3%, 1-methylaspidospermatidine 0.5%, quebrachimine and quebrachit). Leaves contain rhazinilam (lactam).
Air & Water Reactions
Water soluble. Gradually darkens on exposure to air and/or light
Reactivity Profile
Glycerite decomposes at 210° to carbon dioxide and pyrogallol, which can form irritating vapors [USCG, 1999]. Incompatible with salts of heavy metals, alkaloids, gelatin, albumin, starch, oxidizing substances-e.g., permanganates, chlorates; lime water [Merck].
Hazard
Toxic by ingestion and inhalation. Questionable carcinogen.
Fire Hazard
Special Hazards of Combustion Products: Decomposes at 210° to carbon dioxide and pyrogallol, which can form irritating vapors.
Industrial uses
Quebracho is the most common tannic acid derivative widely used in flotation. The
main phenolic nuclei present in Quebracho are resorcinol/phloroglucinol and catechol/pyrogallol.
Quebracho is commercially available in the following three forms: (a) standard
Quebracho, a direct hot-water extract from the heart-wood with adjusted pH (Qu–O), (b)
sulfited Quebracho, in which sulfonic acid groups have been introduced (Qu–S) and (c)
aminated Quebracho (Qu–A), where amine groups are introduced to ordinary Quebracho,
rendering the polymer amphoteric (iso-electric point at pH 7). Each of these types of
Quebracho has a different depressing effect.
Anticancer Research
Tannins, an astringent organic compound, are toxic to many microorganisms due toinhibition of oxidative phosphorylation (Scalbert 1991). It is responsible for thepuckering feeling in the human mouth following the consumption of unripe fruits ortea (Vidal et al. 2004). Pomegranates (Punica granatum) have been widely studiedfor their potent antioxidants, which include ellegitannin, elegiac acid, and punicalagin,which are commonly known for their anti-proliferative and apoptotic effects oncolon cancer cell lines (Seeram et al. 2005). Herbs such as cloves (Syzygium aromaticum)can also play important role in preventing and treating some cancers.Cervical, breast, prostate, and oesophageal cancer cells were killed within 24 h afterthe application of clove oil (Dwivedi et al. 2011). It was also found effective in treatinglung cancer by means of in situ cell proliferation (Banerjee et al. 2006). Tarragon(Artemisia dracunculus) extract is indicated as cytotoxic to breast cancer thoughregulation of certain proteins that induce apoptosis (Obolskiy et al. 2011). Tarragonalso contains potent compounds such as sakuranetin and 6-methoxycapilliarisin thatappear to have anticancer effects on oesophageal cancer by inducing DNA damagein the cancerous cells (Hong and Ying 2015). Cumin (Cuminum cyminum) has beenused since ancient times, and some studies have shown it to have a cytotoxic effectto colorectal cancer cell lines (Prakash and Gupta 2014; Al-Snafi 2016). Thyme(Thymus vulgaris) essential oil has been claimed to demonstrate cytotoxic activity on head and neck squamous cell carcinomas by interrupting reproduction at the transcriptionallevel of cancer cells, with effects such as interfering in N-glycan biosynthesisand extracellular signal-regulated kinase 5 signalling (Sertel et al. 2011).Cinnamon (Cinnamomum sp.) is most commonly known as culinary spice, yet it isalso used for its medicinal properties in treating gastrointestinal sickness. Cinnamonbark extract was evaluated for anticancer ability, and it was found to induce apoptosisin HepG2 cells (liver cancer cells) after 24 h at certain dosages (Varalakshmiet al. 2014). An extract of cinnamon, 2′-hydroxycinnamaldehyde, shows severalantitumour effects on oral cancer cell lines; this is demonstrated by anti-proliferativeactivity of apoptotic cells and inhibition of tumour mass growth (Kim et al. 2010).
Safety Profile
Poison by intravenous and intraperitoneal routes. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
Tannic acid is synthesized by extraction from Nutgalls (Chinese or Turkish), Myrobalans (Terminalia SP.), Oakbark, etc. It is also synthesized by esterification of Glucose with meta-galloylgallic acid.
Environmental Fate
Tannins and tannic acid occur naturally in plants. Essentially all wood and plant tissue contain tannins. Therefore, biodegradation is expected to be the major environmental fate process for tannic acid.
Metabolism
Orally administered Tannic acid is hydrolysable tannin which releases gallic acid and other compounds upon decomposition.
Toxicity evaluation
Tannic acid causes centrilobular liver necrosis following absorption from gastrointestinal tract, mucus membranes, or denuded skin surfaces. Liver metabolism of tannic acid requires methyl-group donors. Therefore, methyl-group donors can be depleted following excessive tannic acid absorption.
Properties of Tannic acid
| Melting point: | 218 °C (lit.) |
| Boiling point: | 862.78°C (rough estimate) |
| Density | 1.2965 (rough estimate) |
| refractive index | 1.7040 (estimate) |
| FEMA | 3042 | TANNIC ACID (QUERCUS SPP.) |
| Flash point: | 198°C |
| storage temp. | Storage temperature: no restrictions. |
| solubility | ethanol: soluble100mg/mL, yellow to brown |
| form | Powder/Solid |
| appearance | light yellow to tan solid |
| color | Yellow to light brown |
| PH | 3.5 (100g/l, H2O, 20°C) |
| Odor | Slight in solution, typical tannic acid |
| Water Solubility | 250 g/L (20 ºC) |
| Sensitive | Air & Light Sensitive |
| Merck | 14,9052 |
| BRN | 8186396 |
| Stability: | Stable. Incompatible with metallic salts, strong oxidizing agents, iron and other heavy metals. |
| IARC | 3 (Vol. 10, Sup 7) 1987 |
| EPA Substance Registry System | Tannic acid (1401-55-4) |
Safety information for Tannic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H303:Acute toxicity,oral |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P403:Store in a well-ventilated place. |
Computed Descriptors for Tannic acid
| InChIKey | LRBQNJMCXXYXIU-PPKXGCFTSA-N |
Tannic acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran







You may like
-
 Tannic Acid CAS 1401-55-4View Details
Tannic Acid CAS 1401-55-4View Details
1401-55-4 -
 Tannic Acid extrapure AR CAS 1401-55-4View Details
Tannic Acid extrapure AR CAS 1401-55-4View Details
1401-55-4 -
 Tannic acid, GR CAS 1401-55-4View Details
Tannic acid, GR CAS 1401-55-4View Details
1401-55-4 -
 Tannic acid 95% CAS 1401-55-4View Details
Tannic acid 95% CAS 1401-55-4View Details
1401-55-4 -
 Tannic acid CAS 1401-55-4View Details
Tannic acid CAS 1401-55-4View Details
1401-55-4 -
 TANNIC ACID Pure CAS 1401-55-4View Details
TANNIC ACID Pure CAS 1401-55-4View Details
1401-55-4 -
 Tannic Acid 60% 850 Molecular weight, Industrial, 75 %View Details
Tannic Acid 60% 850 Molecular weight, Industrial, 75 %View Details
1401-55-4 -
 Tannic Acid (CAS Number: 1401-55-4)View Details
Tannic Acid (CAS Number: 1401-55-4)View Details
1401-55-4
