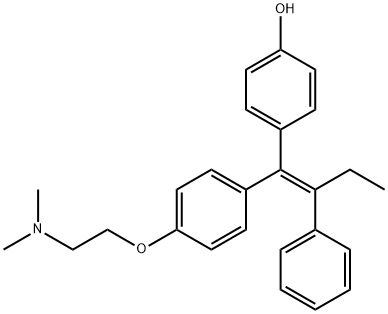
4-HYDROXYTAMOXIFEN
Synonym(s):4-OHT;(Z)-4-Hydroxytamoxifen, 4-OH-TAM, Estrogen Receptor Signaling Regulator II;cis/trans-4-Hydroxytamoxifen;4-(1-[4-(Dimethylaminoethoxy)phenyl]-2-phenyl-1-butenyl)phenol
- CAS NO.:68047-06-3
- Empirical Formula: C26H29NO2
- Molecular Weight: 387.51
- MDL number: MFCD00468090
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-17 16:00:36
What is 4-HYDROXYTAMOXIFEN?
Chemical properties
Powder
The Uses of 4-HYDROXYTAMOXIFEN
A hydroxylated analogue of tamoxifen with anti-estrogenic properties. A metabolite of Tamoxifen.
The Uses of 4-HYDROXYTAMOXIFEN
A metabolite of Tamoxifen (T006000). A hydroxylated analogue of Tamoxifen with anti-estrogenic properties.
The Uses of 4-HYDROXYTAMOXIFEN
(Z)-4-Hydroxy Tamoxifen is a metabolite of Tamoxifen (T006000). (Z)-4-Hydroxy Tamoxifen is a hydroxylated analogue of Tamoxifen. (Z)-4-Hydroxy Tamoxifen is a metabolite of Tamoxifen with anti-estrogenic properties.
The Uses of 4-HYDROXYTAMOXIFEN
tamoxifen metabolite, anti-estrogen, see Tamoxifen 1,01038
What are the applications of Application
(Z)-4-Hydroxytamoxifen is an active metabolite of the antineoplastic agent Tamoxifen and an anti-estrogen agent
Definition
ChEBI: Afimoxifene is a tertiary amino compound that is tamoxifen in which the phenyl group which is in a Z- relationship to the ethyl substituent is hydroxylated at the para- position. It is the active metabolite of tamoxifen. It has a role as an antineoplastic agent, an estrogen receptor antagonist and a metabolite. It is a tertiary amino compound and a member of phenols. It is functionally related to a tamoxifen.
General Description
A cell-permeable, active metabolite of Tamoxifen (Cat. No. 579000) that acts as a potent inhibitor of PKC. It is more potent than the parent compound and inhibits PKC by modifying its catalytic domain. Also available as a 10 mM solution in EtOH (Cat. No. 508225).
Biological Activity
estrogen receptors (er) are members of the superfamily of ligand-modulated nuclear receptors that mediate the actions of steroid hormones, vitamin d, retinoids, and thyroid hormones. er is activated in vivo when bound by naturally occurring estrogens such as 17α-estradiol. in addition to regulating these physiological processes, estrogen also plays a central role in stimulating breast cancer growth. (z)-tamoxifen is a first generation selective er modulators that is currently approved by the fda and is widely used to treat estrogen-dependent breast cancers. its active metabolite, (z)-4-hydroxytamoxifen, is a potent estrogen receptor modulator.
Biochem/physiol Actions
Cell permeable: yes
in vitro
(z)-4-hydroxytamoxifen binds to er with 8-fold higher affinity than tamoxifen. it was found that only the z isomer has the required antiestrogenic activity; the (e)-4-hydroxytamoxifen has only about 5% of its affinity for the er [1].
in vivo
the antioestrogenic activities of (z)-4-hydroxytamoxifen and tamoxifen were determined after oral administration. (z)-4-hydroxytamoxifen was administered to groups of immature rats which also received s.c. injections of 0-2 μg oestradiol. both compounds produced a dose-related decrease in uterine wet weight when compared with the oestradiol-treated controls. at a dose of 1 μg/day, the antiuterotrophic effects of (z)-4-hydroxytamoxifen and tamoxifen were not significantly different but at 5μg/day, (z)-4-hydroxytamoxifen was more active (p < 0.01). (z)-4-hydroxytamoxifen therefore appears to retain its potent antioestrogenic activity after oral administration [2].
storage
-20°C
References
[1] donna d. yu and barry m. forman. simple and efficient production of (z)-4-hydroxytamoxifen, a potent estrogen receptor modulator. j. org. chem. 2003, 68, 9489-9491
[2] jordan vc, collins mm, rowsby l, prestwich g. a monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. j endocrinol. 1977 nov;75(2):305-16.
Properties of 4-HYDROXYTAMOXIFEN
| Melting point: | 105-107°C |
| Boiling point: | 513.45°C (rough estimate) |
| Density | 1.1005 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| solubility | 95% ethanol: 20 mg/mL |
| form | powder |
| pka | 10.35±0.15(Predicted) |
| color | white |
| Stability: | Stable. Store cool. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 68047-06-3 |
Safety information for 4-HYDROXYTAMOXIFEN
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H350:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 4-HYDROXYTAMOXIFEN
| InChIKey | TXUZVZSFRXZGTL-OCEACIFDSA-N |
4-HYDROXYTAMOXIFEN manufacturer
Neugen Labs
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![alpha-[4-[2-(dimethylamino)ethoxy]phenyl]-beta-ethyl-alpha-phenylphenethyl alcohol](https://img.chemicalbook.in/CAS/GIF/748-97-0.gif)
![2-[4-[(E)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethyl-ethanamine](https://img.chemicalbook.in/CAS/GIF/13002-65-8.gif)
You may like
-
 68047-06-3 4-Hydroxytamoxifen 98%View Details
68047-06-3 4-Hydroxytamoxifen 98%View Details
68047-06-3 -
 (Z)-4-Hydroxytamoxifen CAS 68047-06-3View Details
(Z)-4-Hydroxytamoxifen CAS 68047-06-3View Details
68047-06-3 -
 (Z)-4-Hydroxytamoxifen CAS 68047-06-3View Details
(Z)-4-Hydroxytamoxifen CAS 68047-06-3View Details
68047-06-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1