Talc
Synonym(s):Hydrous magnesium silicate
- CAS NO.:14807-96-6
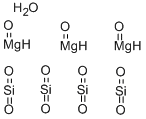
- Empirical Formula: 3MgO.4O2Si.H2O
- Molecular Weight: 379.263
- MDL number: MFCD00676189
- EINECS: 238-877-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
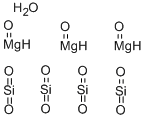
What is Talc?
Absorption
Intended for local use only.
Toxicity
LC50: Not available.
Description
Soapstone is composed primarily of talc, and has been used for most of recorded history as a carving medium; examples thousands of years old are still extant from Egyptian, Assyrian, and Chinese cultures. The term ‘talc’ was first used in AD 869 to describe minerals that were largely composed of what we now know as talc.
Chemical properties
White to almost white micro fine powder, greasy to
Chemical properties
Talc is a very fine, white to grayish-white, odorless, impalpable, unctuous, crystalline powder. It adheres readily to the skin and is soft to the touch and free from grittiness.
Occurrence
Talc is formed under hydrothermal conditions and is a typical mineral of weaker regional metamorphism (regional dynamo-thermometamorphism). It often occurs in association with chlorite, serpentine, or magnesite. The main parent rocks that undergo metamorphic mineral reactions leading to talc formation are either ( magnesite-bearing) siliceous dolomites, or olivine- and/or pyroxene- containing ultrabasics. In ultrabasic rocks, talc is often a product of hydrothermal alteration (autometasomatism). In commercially important deposits, talc occurs in association with tremolite, calcite, quartz, and dolomite, as the product of a more intense regional metamorphism of siliceous dolomites, and with forsterite and anthophyllite due to intense regional metamorphic overprint of ultrabasics. However, talc can also be observed as an authigenic new formation, e.g., in sandy sediments and salt deposits. Other minerals that occur in association with talc include chlorites, mica, actinolite, feldspars, rutile, pyrrhotite, pyrite, magnetite, and hematite. Limonite, a product of the weathering of iron- containing minerals, especially iron- containing ore minerals, can often be found interspersed with talc.
History
Talc, soapstone, and steatite have been used by humans as raw materials since prehistoric times. Molds carved from soapstone were used in the Bronze Age and early Iron Age for casting weapons and tools. In the Mediterranean cultures of the classical period, stone carvings were made from soapstone, and talc was used for treating wounds and in the production of cosmetic powder. In ancient Rome, women used large amounts of powder and rouge. The properties of talc, especially its characteristic greasy feel, were described by Pliny the Elder. The old Arabic word talq, which indicates its greasy nature, gave its name to the mineral. In 1550, Catherin de Medici made it once again fashionable to use facial makeup in the form of powdered talc colored by the addition of pigments, a fashion that found innumerable imitators and has continued without interruption until today.
The Uses of Talc
Talc is a mineral composed of hydrated magnesium silicate with the chemical formula H2Mg3(SiO3)4 or Mg3Si4O10(OH)2. Talc is used in many industries such as paper making, plastic, paint and coatings, rubber, food, electric cable, pharmaceuticals, cosmetics
The Uses of Talc
Dusting powder, either alone or with starch or boric acid, for medicinal and toilet Preparations; excipient and filler for pills, tablets and for dusting tablet molds; clarifying liquids by filtration. As pigment in paints, varnishes, rubber; filler for paper, rubber, soap; in fireproof and cold-water paints for wood, metal and stone; lubricating molds and machinery; glove and shoe powder; electric and heat insulator.
The Uses of Talc
talc adds softness and sliding ability to a cosmetic formulation. It is also used as a bulking and opacifying agent, and as an absorbent in makeup preparations. Talc is an inert powder, generally made from finely ground magnesium silicate, a mineral.
Background
Talc is a mineral composed primarily of magnesium, silicon and oxygen. It is a substance often found in cosmetic and personal hygiene products including baby powder, adult body powders and facial powders. Talc helps absorb moisture, freshen materials, and reduce friction to prevent rashes.
Although talc has been widely used for decades, lawsuits that have come to the surface claim serious health complications linked to its use. In fact, recent studies have reported that talc increases the risk of ovarian and endometrial cancers by approximately 30 percent.
Indications
Indicated to prevent recurrence of malignant pleural effusions in symptomatic patients during thoracoscopy or open thoracotomy.
Production Methods
Talc is a naturally occurring hydropolysilicate mineral found in
many parts of the world including Australia, China, Italy, India,
France, and the USA.
The purity of talc varies depending on the country of origin. For
example, Italian types are reported to contain calcium silicate as the
contaminant; Indian types contain aluminum and iron oxides;
French types contain aluminum oxide; and American types contain
calcium carbonate (California), iron oxide (Montana), aluminum and iron oxides (North Carolina), or aluminum oxide (Alabama).
Naturally occurring talc is mined and pulverized before being
subjected to flotation processes to remove various impurities such as
asbestos (tremolite); carbon; dolomite; iron oxide; and various
other magnesium and carbonate minerals. Following this process,
the talc is finely powdered, treated with dilute hydrochloric acid,
washed with water, and then dried. The processing variables of
agglomerated talc strongly influence its physical characteristics.
Definition
Talc, a hydrated magnesium sulfate, is the primary component of most face powders (eye shadows and blushers). In some products, talc makes up to 70% of the formulation. Cosmetic talc should be white, free of asbestos, should have high spreadability or slip, with low coverage. Particle size is acceptable if the material passes through a 200 mesh sieve. Micronized talc is generally lighter and fluffier but less smooth on the skin than regular grades. Typically talc products are sterilized using gamma irradiation. Cosmetic talcs are mined in Italy, France, Norway, India, Spain, China, Egypt, Japan, and the United States. Talc is fairly hydrophobic, although treatments are used to enhance its texture.
General Description
Odorless white to grayish-white very fine crystalline powder (unctuous). Readily adheres to the skin. Nonflammable, noncombustible, and nontoxic.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Talc has low reactivity.
Health Hazard
Pure talc is toxicologically harmless. However, where there are high concentrations of dust in the air, face masks should be worn. If the talc contains detectable amounts of asbestos or asbestos minerals, an MAK value of 2.0 mg/m3 applies. Talc is a nontoxic, inert substance or raw material, but it can contaminate wounds and if inhaled it can cause lung irritations.
Fire Hazard
Literature sources indicate that Talc is nonflammable.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Talc was once widely used in oral solid dosage formulations as a
lubricant and diluent, although today it is less
commonly used. However, it is widely used as a dissolution
retardant in the development of controlled-release products. Talc is also used as a lubricant in tablet formulations; in a novel
powder coating for extended-release pellets; and as an adsorbant.
In topical preparations, talc is used as a dusting powder,
although it should not be used to dust surgical gloves. Talc is a natural material; it may therefore frequently contain
microorganisms and should be sterilized when used as a dusting
powder.
Talc is additionally used to clarify liquids and is also used in
cosmetics and food products, mainly for its lubricant properties.
Safety Profile
The talc with less than 1 percent asbestos is regarded as a nuisance dust. Talc with greater percentage of asbestos may be a human carcinogen. A human skin irritant. Prolonged or repeated exposure can produce a form of pulmonary fibrosis (talc pneumoconiosis) which may be due to asbestos content. Questionable carcinogen with experimental tumorigenic data. A common air contaminant.
Safety
Talc is used mainly in tablet and capsule formulations. Talc is not
absorbed systemically following oral ingestion and is therefore
regarded as an essentially nontoxic material. However, intranasal or
intravenous abuse of products containing talc can cause granulomas
in body tissues, particularly the lungs. Contamination of
wounds or body cavities with talc may also cause granulomas;
therefore, it should not be used to dust surgical gloves. Inhalation of
talc causes irritation and may cause severe respiratory distress in
infants.
Although talc has been extensively investigated for its carcinogenic
potential, and it has been suggested that there is an increased
risk of ovarian cancer in women using talc, the evidence is
inconclusive. However, talc contaminated with asbestos has
been proved to be carcinogenic in humans, and asbestos-free grades
should therefore be used in pharmaceutical products.
Also, long-term toxic effects of talc contaminated with large
quantities of hexachlorophene caused serious irreversible neurotoxicity
in infants accidentally exposed to the substance.
Carcinogenicity
In vitro assay of a number of respirable talc specimens of high purity demonstrated a modest but consistent cytotoxicity to macrophages; the investigators conclude that the talcs would be expected to be slightly fibrogenic in vivo.
Metabolism
Intended for local use only.
storage
Talc is a stable material and may be sterilized by heating at 160°C
for not less than 1 hour. It may also be sterilized by exposure to
ethylene oxide or gamma irradiation.
Talc should be stored in a well-closed container in a cool, dry
place.
Toxicity evaluation
The mechanism by which the talc is toxic is largely physical in its function, impairing organ function by inhibiting necessary movement or transfer of material. Pulmonary responses can occur through production of microemboli and granuloma formation.
Incompatibilities
Incompatible with quaternary ammonium compounds.
Regulatory Status
Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (buccal tablets; oral capsules and tablets; rectal and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Talc
| Melting point: | 800 °C |
| Density | 2.7-2.8 |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Practically insoluble in water, in ethanol (96 per cent) and in dilute solutions of acids and alkali hydroxides. |
| form | Powder/Solid |
| color | White to pale gray |
| Odor | at 100.00?%. odorless |
| Water Solubility | Insoluble in water, cold acids, alkalies. |
| Merck | 14,9037 |
| Exposure limits | ACGIH: TWA 2 mg/m3 NIOSH: IDLH 1000 mg/m3; TWA 2 mg/m3 |
| IARC | (Vol. 42, Sup 7) 1987, 3 (Vol. 42, Sup 7, 93) 2010, 2B (Vol. 93) 2010 |
| EPA Substance Registry System | Talc (14807-96-6) |
Safety information for Talc
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for Talc
| InChIKey | FPAFDBFIGPHWGO-UHFFFAOYSA-N |
Talc manufacturer
TIRUMALA INORGANICS
Tara Minerals and Chemicals Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Purified talcum powder supplierView Details
Purified talcum powder supplierView Details
14807-96-6 -
 TALC 99%View Details
TALC 99%View Details -
 Magnesium silicate monohydrate (Talc) CAS 14807-96-6View Details
Magnesium silicate monohydrate (Talc) CAS 14807-96-6View Details
14807-96-6 -
 Magnesium silicate monohydrate (Talc) CAS 14807-96-6View Details
Magnesium silicate monohydrate (Talc) CAS 14807-96-6View Details
14807-96-6 -
 TalcumPowder 99%View Details
TalcumPowder 99%View Details -
 Talc 98%View Details
Talc 98%View Details -
 Magnesium silicate monohydrate (Talc) CAS 14807-96-6View Details
Magnesium silicate monohydrate (Talc) CAS 14807-96-6View Details
14807-96-6 -
 Talc Powder Fine ExiPlus CAS 14807-96-6View Details
Talc Powder Fine ExiPlus CAS 14807-96-6View Details
14807-96-6