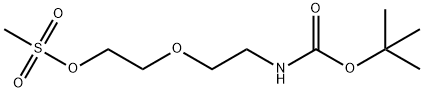
Br-PEG1-NHBoc
- CAS NO.:164332-88-1
- Empirical Formula: C9H18BrNO3
- Molecular Weight: 268.15
- MDL number: MFCD24167474
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
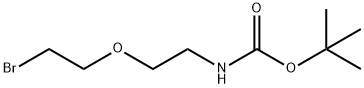
What is Br-PEG1-NHBoc?
Description
t-boc-N-amido-PEG2-bromide is a PEG derivative containing a bromide group and Boc-protected amino group. The hydrophilic PEG spacer increases solubility in aqueous media. The bromide (Br) is a very good leaving group for nucleophilic substitution reactions. The Boc group can be deprotected under mild acidic conditions to form the free amine.
The Uses of Br-PEG1-NHBoc
t-boc-N-amido-PEG2-bromide can be used in medical research, drug-release, nanotechnology and new materials research, cell culture. In the study of ligand, polypeptide synthesis support, a graft polymer compounds, new materials, and polyethylene glycol-modified functional coatings and other aspects of the active compound.
The Uses of Br-PEG1-NHBoc
tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate is a cleavable ADC linker for the synthesis of antibody drug conjugates (ADCs).
Synthesis
The synthesis of tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate is as follows:
Alcohol 64 (1 .25 g, 6.09 mmol) was dissolved in DCM (34 mL). The solution was cooled to 0°C and methanesulfonyl chloride (MsCI, 0.80 mL, 10.3 mmol, 1 .7 equiv) was added to the solution followed by Et3N (1.9 mL, 13.4 mmol, 2.2 equiv). After stirring for 3 h at rt, the reaction mixture was diluted with acetone (33 mL) and LiBr (8.9 g, 103 mmol, 17 equiv) was added. The reaction mixture was stirred overnight at rt. After that time, the solvents were evaporated under reduced pressure. The crude residue was diluted with EtOAc and washed with H20 and brine. The organic phase was dried over anhyd Na2S04. The suspension was filtered over cotton and the filtrate concentrated in vacuo. Purification by flash chromatography eluting with PE/Acetone (85:15→ 8:2) gave tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate as a colourless oil.
Biological Activity
The chemical structure of tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate consists of a tert-butyl carbamate group attached to a N-methylcarbamate moiety via a 2-(2-bromoethoxy)ethyl linker. This unique structure contributes to its potential biological activity. The compound has been found to exhibit inhibitory effects on specific biological targets, primarily through its mechanism of action involving the disruption of cell function and signal transduction pathways. The potency of tert-butyl N-[2-(2-bromoethoxy)ethyl]-N-methylcarbamate in modulating these targets varies depending on the specific biological system under investigation.
Biotechnological Applications
The biological effects of tert-Butyl (2-(2-bromoethoxy)ethyl)carbamate on cell function and signal transduction have been extensively studied. It could interfere with various cellular processes, including cell proliferation, apoptosis, and differentiation. Additionally, the compound has demonstrated potential therapeutic effects in certain disease models, highlighting its potential as a drug candidate. However, it is important to consider the potential toxic effects of tert-butyl N-[2-(2-bromoethoxy)ethyl]-N-methylcarbamate, as excessive exposure or misuse may lead to adverse health outcomes.
Properties of Br-PEG1-NHBoc
| Boiling point: | 336.6±22.0 °C(Predicted) |
| Density | 1.283±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in Water, DMSO, DCM, DMF |
| pka | 12.19±0.46(Predicted) |
| form | Liquid |
| color | Colorless to light yellow |
Safety information for Br-PEG1-NHBoc
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Br-PEG1-NHBoc
New Products
Ethyl 3,3- diethoxypropionate 7-Bromo-2-chloroquinoxaline N-Benzyl-3-hydroxypiperidine 2-(4-bromophenyl)-2-methylpropanoic acid Benzylacetoacetate Radiator Flux Zinc Chloride Solution (All Grades) Phenylazomalononitrile 2-Fluoro-6-iodobenzoic acid 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 2,3-Difluoro-6-methoxybenzyl Chloride 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane Ethyl2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]quinoxaline-8(7H)-carboxylate Acetyl-meldrum's acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid N Ethylmethylamine Ethyl Methanesulfonate N N' DimethylEthylenediamine Lead II BromideRelated products of tetrahydrofuran


![O-[2-(Boc-amino)ethyl]-Oμ-(2-carboxyethyl)polyethylene glycol 3,000](https://img.chemicalbook.in/CAS/20180714/GIF/187848-68-6.gif)
You may like
-
![164332-88-1 TERT-BUTYLN-[2-(2-BROMOETHOXY)ETHYL]CARBAMATE 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/2333a637-134d-494f-8ac9-f536a06cf1f2.png) 164332-88-1 TERT-BUTYLN-[2-(2-BROMOETHOXY)ETHYL]CARBAMATE 98%View Details
164332-88-1 TERT-BUTYLN-[2-(2-BROMOETHOXY)ETHYL]CARBAMATE 98%View Details
164332-88-1 -
![tert-Butyl n-[2-(2-bromoethoxy)ethyl]carbamate 95% CAS 164332-88-1](https://img.chemicalbook.in//Content/image/CP5.jpg) tert-Butyl n-[2-(2-bromoethoxy)ethyl]carbamate 95% CAS 164332-88-1View Details
tert-Butyl n-[2-(2-bromoethoxy)ethyl]carbamate 95% CAS 164332-88-1View Details
164332-88-1 -
![4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%](https://img.chemicalbook.in//Content/image/CP5.jpg) 4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
4-chloro-7H-pyrrolo [2,3-d]pyrimidine 3680-69-1 98%View Details
3680-69-1 -
 4'-Benzyloxy-2-bromopropiophenone 98%View Details
4'-Benzyloxy-2-bromopropiophenone 98%View Details
35081-45-9 -
 53928-30-6 98%View Details
53928-30-6 98%View Details
53928-30-6 -
 5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 2-Amino-3-(1,2-dihydro-2-oxoquinoline-4-yl)propanoic acid 97%View Details
5162-90-3 -
 4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
4-(4-Chlorobenzyl)-2-(1-methylazepan-4-yl)phthalazin-1(2H)-one hydrochloride 98 %View Details
79307-93-0 -
 (R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
(R)-2-amino-N-benzyl-3-methoxypropanamide 98%View Details
196601-69-1
