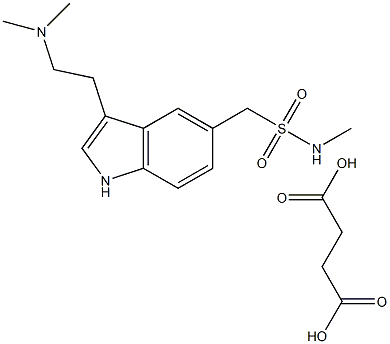
SuMatriptan N-Oxide
- CAS NO.:212069-94-8
- Empirical Formula: C14H21N3O3S
- Molecular Weight: 311.4
- MDL number: MFCD24386518
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 15:12:45
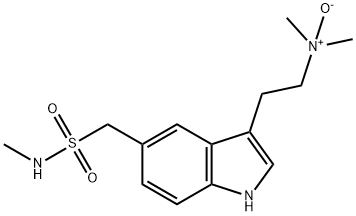
What is SuMatriptan N-Oxide?
The Uses of SuMatriptan N-Oxide
Sumatriptan N-Oxide (Sumatriptan EP Impurity D) is the degradation product of the antimigraine drug Sumatriptan (S810000).
The Uses of SuMatriptan N-Oxide
A degradation product of the antimigraine drug Sumatriptan (S810000).
Properties of SuMatriptan N-Oxide
| Melting point: | 190-193 °C(Solv: acetone (67-64-1)) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Very Slightly, Heated), Methanol (Very Slightly, Heated), Water (Slightly) |
| form | Solid |
| pka | 11.28±0.40(Predicted) |
| color | Pale Beige to Light Brown |
| Stability: | Hygroscopic, Unstable in DMSO |
Safety information for SuMatriptan N-Oxide
Computed Descriptors for SuMatriptan N-Oxide
SuMatriptan N-Oxide manufacturer
Clickchem Research LLP
2Y
Phone:+91-8140031133
Whatsapp: +91- 8140031133
product: 212069-94-8 SUMATRIPTAN N-OXIDE 90 % Above
Allas Chem Technologies Pvt Ltd
1Y
Phone:+91-9902524108
Whatsapp: +91-9902524108
product: 212069-94-8 97%
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![[3-[2-(diMethylaMino)ethyl]-2-[[3-[2-(diMethylaMino)ethyl]-1H-indol-5-yl]Methyl]-1H-indol-5-yl]-N-MethylMethanesulfonaMide, succinate salt](https://img.chemicalbook.in/CAS/GIF/545338-89-4.gif)
You may like
-
 212069-94-8 98%View Details
212069-94-8 98%View Details
212069-94-8 -
 212069-94-8 97%View Details
212069-94-8 97%View Details
212069-94-8 -
 212069-94-8 SUMATRIPTAN N-OXIDE 90 % AboveView Details
212069-94-8 SUMATRIPTAN N-OXIDE 90 % AboveView Details
212069-94-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.
