Sumatriptan succinate
Synonym(s):3-[2-(Dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide succinate;GR-43175;Sumatriptan succinate
- CAS NO.:103628-48-4
- Empirical Formula: C18H27N3O6S
- Molecular Weight: 413.48848
- MDL number: MFCD00902856
- EINECS: 600-463-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
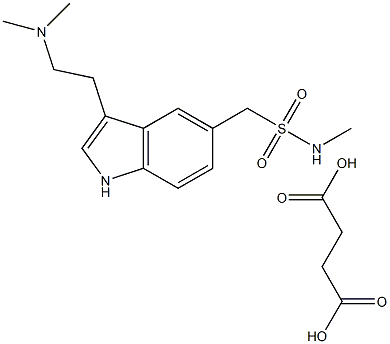
What is Sumatriptan succinate?
Description
Sumatriptan succinate is a highly selective 5HT1-like receptor agonist introduced as a new treatment for migraine. It is indicated for the acute relief of migraine and cluster headache. Oral administration is reported to be free of substantial side effects. The compound appears to be a significant advance over the use of ergotamine and other agents in the treatment of migraine.
Chemical properties
White Crystalline Powder
Originator
Glaxo (United Kingdom)
The Uses of Sumatriptan succinate
Sumatriptan Succinate is a selective 5-HT1B/1D-receptor agonist with anticonvulsant properties (1,2). Sumatriptan Succinate is used for migraine relief (2).
The Uses of Sumatriptan succinate
Sumatriptan (succinate) is a serotonin 5-hydroxytryptamine 1 (5-HT1) receptor agonist with selective affinity for 5-HT1B and 5-HT1D receptors with IC50 values of 9.3 and 7.3 nM, respectively. It also has affinity for the 5-HT1F receptor (IC50 = 17.8 nM). Sumatriptan (succinate) has been shown to induce vasoconstriction of human middle meningeal arteries (EC50 = 89.9 nM) and reduce vascular inflammation associated with migraines.
Definition
ChEBI: A succinate salt obtained by reaction of sumatriptan with one equivalent of succinic acid. Selective agonist for a vascular 5-HT1 receptor subtype (probably a member of the 5-HT1D family). Used for the acute treatm nt of migraine with or without aura in adults.
Manufacturing Process
A solution of (phenylthio)acetaldehyde (6.05 g) in absolute ethanol (180 ml)
was added over 10 min to a solution of 4-hydrazino-Nmethylbenzenemethanesulphonamide
hydrochloride (10 g) in water (180 ml)
with cooling. After addition of the aldehyde was complete, the mixture was
stirred at 5°C for a period of 14 h. The precipitated solid was filtered off,
washed with water (200 ml), hexane (200 ml) and dried in vacuo to give the
N-methyl-4-[2-[2-(phenylthio)ethylidene]hydrazino]benzenemethanesulphonamide
(10.95 g), melting point 110°-112°C.
A solution of the N-methyl-4-[2-[2-
(phenylthio)ethylidene]hydrazino]benzenemethane-sulphonamide in absolute
ethanol (300 ml) was saturated with hydrogen chloride gas (ca. 30 min) whilst
being cooled in an ice-water bath, allowed to stir at room temperature for 3 h
and filtered. The filtrate was concentrated in vacuo and chromatographed to
afford a foam, which solidified on trituration with ether to an amorphous
powder (2.17 g). A sample was recrystallized from hexane-dichloromethane to
give the N-methyl-3-(phenylthio)-1H-indole-5-methanesulphonamide, melting
point 133°-134°C.
To a solution of N-methyl-3-(phenylthio)-1H-indole-5-methanesulphonamide
(460 mg) in absolute ethanol (50 ml) was added Raney nickel [4.6 g, 50%
slurry in water, washed to neutrality with deionized water (60 ml)] and the
reaction mixture refluxed for 16 h under an atmosphere of nitrogen. On cooling to room temperature, the supernatant was removed and the Raney
nickel extracted with ethanol (2x50 ml, which was brought to a gentle reflux
for 15 min under an atmosphere of nitrogen). The combined extracts were
filtered through a sand-celite pad and concentrated in vacuo. Chromatography
of the residue, afforded an oil (87 mg) which crystallized from ether-hexane
to give the N-methyl-1H-indole-5-methanesulphonamide (90 mg), melting
point 127°-129°C.
To N,N-diethyl chloroacetamide (800 mg) at 0°C was added phosphorous
oxychloride (250 μl) over a period of 30 sec. After the addition was complete,
the mixture was allowed to stir at 0°C for 15 min and then at room
temperature for 20 min. The N-methyl-1H-indole-5-methanesulphonamide
(300 mg) was added at 0°C and the mixture warmed to 65°C, whereupon it
dissolved. The mixture was stirred for 2 h at this temperature then poured
onto ice (ca. 5 g) and chloroform (5 ml) and stirred vigorously for 1 h. A solid
was filtered off, washed with water (50 ml), and hexane (100 ml) and dried in
vacuo to give the 3-(chloroacetyl)-N-methyl-1H-indole-5-
methanesulphonamide (192 mg).
A solution of the 3-(chloroacetyl)-N-methyl-1H-indole-5-
methanesulphonamide (160 mg) in ethanolic dimethylamine (30 ml, 33% w/v
solution in ethanol) was heated to reflux for 2 h. On cooling to room
temperature the solvent was removed in vacuo and the residue was
chromatographed to afford the 3-[(dimethylamino)acetyl]-N-methyl-1Hindole-
5-methanesulphonamide, melting point 230°C, dec.
To a suspension of the 3-[(dimethylamino)acetyl]-N-methyl-1H-indole-5-
methanesulphonamide (46.5 mg) in 1-propanol (5 ml) was added sodium
borohydride (62 mg). The reaction mixture was brought to reflux for a period
of 3 h, then an additional quantity of borohydride (60 mg) was added. After
refluxing for a further 1 h, the mixture was allowed to cool to room
temperature and quenched with 2 N HCl (10 ml). The aqueous solution was
washed with ethyl acetate (5 ml) then neutralized (NaHCO3 solution) and
extracted with ethyl acetate (3 x 15 ml). The combined extracts were
concentrated in vacuo and the residue chromatographed to give the 3-[2-
(dimethylamino)ethyl]-N-methyl 1H-indole-5-methanesulphonamide as a gum
(2 mg) which was shown by TLC.
Succinic acid in hot methanol was added to a hot solution of the the 3-[2-
(dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulphonamide in
absolute ethanol and the mixture was heated to reflux with stirring to give a
solution. The solution was allowed to cool with stirring to room temperature,
and the resultant suspension was farther cooled in an ice-bath for 2 h. The
solid was filtered off, washed with ethanol, and dried in vacuo to give the 3-
[2-(dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulphonamide, salt
with succinic acid (1:1).
brand name
Imitrex (GlaxoSmithKline);Imigran.
Therapeutic Function
Serotoninergic
World Health Organization (WHO)
Suprofen, a nonsteroidal anti-inflammatory agent, was introduced in 1983 for use as an analgesic for the symptomatic relief of mild to moderate pain and for primary dysmenorrhoea. By 1986 it had become evident that its use was occasionally associated with flank pain sometimes accompanied by evidence of decreased renal function. The Arthritis Advisory Committee of the United States Food and Drug Administration met in December 1986 to review the situation and decided against withdrawing suprofen from the market. However, in May 1987 the Committee for Proprietary Medicinal Products of the European Community recommended that all marketing authorizations should be suspended. The manufacturer subsequently decided to suspend sale worldwide on the grounds that sales had diminished to the point where the product was no longer economically viable.
Biological Activity
Selective 5-HT 1 receptor agonist (K i values are 17, 27 and 100 nM at 5-HT 1D , 5-HT 1B and 5-HT 1A receptors respectively). Antimigraine agent.
Biochem/physiol Actions
Sumatriptan succinate is a 5-HT1 serotonin receptor agonist.
Storage
+4°C
Properties of Sumatriptan succinate
| Melting point: | 165-166°C |
| storage temp. | -20°C |
| solubility | H2O: >20mg/mL |
| form | solid |
| color | white to off-white |
| Merck | 14,8997 |
| CAS DataBase Reference | 103628-48-4(CAS DataBase Reference) |
Safety information for Sumatriptan succinate
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Sumatriptan succinate
| InChIKey | PORMUFZNYQJOEI-UHFFFAOYSA-N |
Sumatriptan succinate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![[3-[2-(diMethylaMino)ethyl]-2-[[3-[2-(diMethylaMino)ethyl]-1H-indol-5-yl]Methyl]-1H-indol-5-yl]-N-MethylMethanesulfonaMide, succinate salt](https://img.chemicalbook.in/CAS/GIF/545338-89-4.gif)
You may like
-
 Sumatriptan Succinate 98%View Details
Sumatriptan Succinate 98%View Details -
 Sumatriptan Succinate 98%View Details
Sumatriptan Succinate 98%View Details -
 Sumatriptan succinate >98% (HPLC) CAS 103628-48-4View Details
Sumatriptan succinate >98% (HPLC) CAS 103628-48-4View Details
103628-48-4 -
 Sumatriptan succinate 95.00% CAS 103628-48-4View Details
Sumatriptan succinate 95.00% CAS 103628-48-4View Details
103628-48-4 -
 Sumatriptan Succinate CAS NO: 103628-46-2 SPIL, Grade Standard: USPView Details
Sumatriptan Succinate CAS NO: 103628-46-2 SPIL, Grade Standard: USPView Details
103628-46-2 -
 Sumatriptan Succinate PowderView Details
Sumatriptan Succinate PowderView Details
103628-48-4 -
 Sumatriptan Succinate API MANUFACTURER INDIAView Details
Sumatriptan Succinate API MANUFACTURER INDIAView Details
103628-48-4 -
 Sumatriptan Succinate ApiView Details
Sumatriptan Succinate ApiView Details
103628-48-4
