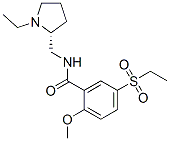
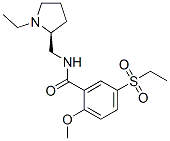
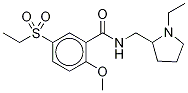
Sultopride
- CAS NO.:53583-79-2
- Empirical Formula: C17H26N2O4S
- Molecular Weight: 354.468
- MDL number: MFCD00865418
- EINECS: 2586419
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
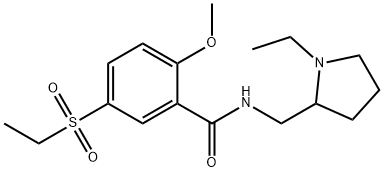
What is Sultopride?
Originator
Barnetil,Delagrange,France,1976
Background
Sultopride is used in Japan, Hong Kong, and Europe to treat schizophrenia. It is of the drug class atypical antipsychotics .
Definition
ChEBI: Sultopride is a member of salicylamides.
Manufacturing Process
A solution of 17.22 g of N-ethyl-α-aminomethylpyrrolidine in 360 ml of
pyridine is placed in a 1 l balloon flask. A solution of 3.51 g of phosphorus
trichloride in 40 ml of pyridine is added at ambient temperature. After the
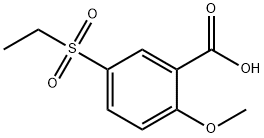
mixture has been stirred for 1 hour, 10 g of 2-methoxy-5-ethylsulfonylbenzoic
acid is introduced. The mixture is heated under reflux for 4 ? hours. After
cooling, the solvent is evaporated under vacuum and the residue is dissolved
in 200 ml of 20% sodium hydroxide. The solution is extracted with 200 ml of
chloroform.
The organic solution is dried and filtered and the solvent is evaporated under
vacuum; the residue is dissolved in 150 ml of ethanol and the solution is
acidified with hydrochloric acid. The hydrochloride is dried without heating and
recrystallized from 100 ml of absolute ethanol. 7.2 g of N-(1-ethyl-2-
pyrrolidyl-methyl-2-methoxy-5-ethylsulfonylbenzamide hydrochloride is
produced. Melting point: 190°C to 193°C.
brand name
Banotil;Barnotil;Topral.
Therapeutic Function
Neuroleptic
Metabolism
Not Available
Properties of Sultopride
| Melting point: | 181-182 °C |
| Boiling point: | 530.0±50.0 °C(Predicted) |
| Density | 1.1814 (rough estimate) |
| refractive index | 1.6930 (estimate) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 13.99±0.46(Predicted) |
Safety information for Sultopride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sultopride
Sultopride manufacturer
Sibram Pharmaceutical
Elam Pharma Pvt Ltd
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran
You may like
-
![53583-79-2 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulfonyl-2-methoxybenzamid 99%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/e8a4b4b0-834e-480b-83d8-78f03d1b0160.png) 53583-79-2 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulfonyl-2-methoxybenzamid 99%View Details
53583-79-2 4-amino-N-[(1-ethylpyrrolidin-2-yl)methyl]-5-ethylsulfonyl-2-methoxybenzamid 99%View Details
53583-79-2 -
 53583-79-2 98%View Details
53583-79-2 98%View Details
53583-79-2 -
 Sultopride 53583-79-2 99%View Details
Sultopride 53583-79-2 99%View Details
53583-79-2 -
 53583-79-2 Sultopride 98%View Details
53583-79-2 Sultopride 98%View Details
53583-79-2 -
 Diethyl Disulfide 99% HPLCView Details
Diethyl Disulfide 99% HPLCView Details
110-81-6 -
 6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. -
 4-chloro-3,5-dinitropyridine 98%View Details
4-chloro-3,5-dinitropyridine 98%View Details -
 171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1