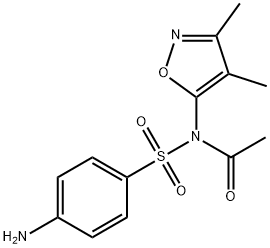
SULFISOXAZOLE ACETYL (200 MG)
Synonym(s):N1-Acetylsulfisoxazole
- CAS NO.:80-74-0
- Empirical Formula: C13H15N3O4S
- Molecular Weight: 309.3409
- MDL number: MFCD00072139
- EINECS: 2013053
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is SULFISOXAZOLE ACETYL (200 MG)?
Absorption
Readily absorbed from the gastrointestinal tract .
In a pharmacokinetic study, the adjustments for variable renal clearances between oral and intravenous administration and using the unbound plasma concentrations, the bioavailability for an oral dose of sulfisoxazole was found to be 0.95 +/- 0.04 .
Toxicity
LD50=6800 mg/kg (Orally in mice) .
Sulfonamide-induced hypersensitivity reactions, although uncommon, can be severe. They can include rare life-threatening cutaneous reactions such as erythema multiform (Stevens-Johnson syndrome) and toxic epidermal necrolysis. Crystalluria may result in dysuria, renal colic, haematuria and acute renal obstruction .
Other infrequent reactions include granulocytopenia, agranulocytosis, aplastic anemia, thrombocytopenic purpura and toxic hepatitis. Rarely, hemolysis may occur in individuals deficient in glucose-6-phosphate dehydrogenase .
The severe or irreversible adverse effects of sulfisoxazole, which give rise to more complications which may include: nephrotoxicity, blood dyscrasias, interstitial nephritis, hematuria, crystalluria, oliguria, anuria, lumbar pain, and tubular necrosis .
The symptomatic adverse reactions produced by sulfisoxazole are more or less tolerable and if severe, may be treated symptomatically, these include anorexia, diarrhea, rashes, pruritus, nausea and vomiting, hypersensitivity reactions, Photosensitivity reactions .
Overdosage: Continuously forced diuresis may be beneficial and an alkaline urine should be initiated .
Originator
Gantrisin Acetyl ,Roche ,US ,1954
The Uses of SULFISOXAZOLE ACETYL (200 MG)
Antibacterial.
The Uses of SULFISOXAZOLE ACETYL (200 MG)
Sulfisoxazole Acetyl is an antibiotic used in the prevention of infection especially when used in conjunction with erythromycin.
Background
Sulfisoxazole acetyl is an ester of sulfisoxazole, a broad-spectrum sulfanilamide and a synthetic analog of para-aminobenzoic acid (PABA) with antibacterial activity. Sulfisoxazole acetyl competes with PABA for the bacterial enzyme, dihydropteroate synthase, preventing the incorporation of PABA into dihydrofolic acid, which is the precursor of folic acid. This process causes an inhibition of bacterial folic acid synthesis and de novo synthesis of purines and pyrimidines, resulting in cell growth arrest and cell death .
It is often combined with erythromycin to treat acute otitis media caused by the bacteria, haemophilus influenzae .
Indications
Acute, recurrent or chronic urinary tract infections (primarily pyelonephritis, pyelitis and cystitis) due to susceptible organisms (usually Escherichia coli, Klebsiella-Enterobacter, staphylococcus, Proteus mirabilis and, less frequently, Proteus vulgaris) in the absence of obstructive uropathy or foreign bodies
Meningococcal meningitis where the organism has been demonstrated to be susceptible. Haemophilus influenzae meningitis as adjunctive therapy with parenteral streptomycin
Meningococcal meningitis prophylaxis .
Acute otitis media due to Haemophilus influenzae when used concomitantly with adequate doses of penicillin or erythromycin (see appropriate labeling for prescribing information) .
Trachoma, inclusion conjunctivitis, nocardiosis, chancroid, toxoplasmosis as adjunctive therapy with pyrimethamine. Malaria due to chloroquine-resistant strains of Plasmodium falciparum, when used as adjunctive therapy .
Currently, the increasing frequency of resistant organisms is a limitation of the usefulness of antibacterial agents including the sulfonamides, especially in the treatment of chronic and recurrent urinary tract infections .
Definition
ChEBI: Sulfisoxazole acetyl is a sulfonamide and a member of benzenes.
Manufacturing Process
267 grams (1 mol) of sulfisoxazole were suspended in 400 ml of acetone and
79 grams (1 mol) of dry pyridine at 20-25°C in a round-bottom flask equipped
with a stirrer and thermometer. 132 grams (1 mol) of acetic anhydride were
added within 3 minutes with stirring. The sulfisoxazole dissolved in the
mixture and a clear solution resulted. The temperature rose to 39-40°C. After
stirring for several minutes, the product started to crystallize as a white
crystalline mush. The temperature rose to 42-43°C maintained itself at this
temperature for 15-30 minutes, and then started to drop. Stirring was
continued for 5 hours and the mixture was then allowed to stand for 10 hours.
One liter of 2.5-3.0% ice-cold aqueous ammonia and some fresh ice were
then added while stirring and the crystals were filtered without delay. The
crystals were washed on the filter with 1 liter of ice-cold 1% ammonia and
then with 1 liter of water. The material on the filter was well pressed off,
washed with 200-300 ml of alcohol and dried at 70°C to constant weight. The
N-monoacetyl sulfisoxazole melted at 193-194°C and showed a positive
Bratton-Marshall reaction and a positive Hucknall-Turfat reaction.
The product is in the form of colorless crystals which are somewhat water
repellent. It is insoluble in alkali but is saponified upon standing in alkaline
suspension (3% ammonia). It is soluble in strong acids (20-36% HCl or 10 N
H2SO4) and is rapidly saponified upon standing.
brand name
Gantrisin (Roche).
Therapeutic Function
Antimicrobial
General Description
Sulfisoxazole acetyl shares the actions and usesof the parent compound, sulfisoxazole. The acetyl derivativeis tasteless and, therefore, suitable for oral administration, especiallyin liquid preparations. The acetyl compound is splitin the intestinal tract and absorbed as sulfisoxazole; that is, itis a prodrug for sulfisoxazole.
Pharmacokinetics
Sulfisoxazole is a sulfonamide antibiotic. The sulfonamides are synthetic bacteriostatic antibiotics with action against most gram-positive and many gram-negative organisms. Many strains of an individual species may be resistant to this drugf. Sulfonamides inhibit the multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus .
Metabolism
Sulfisoxazole acetyl is a prodrug of sulfisoxazole. The acetyl group is added to make the drug poorly water-soluble and is hydrolyzed in vivo to the active drug , .
N1-acetyl sulfisoxazole is metabolized to sulfisoxazole by digestive enzymes in the gastrointestinal tract and is absorbed as sulfisoxazole. This enzymatic splitting is thought to be responsible for slower absorption and lower peak blood concentrations are achieved after administration of an equal oral dose of sulfisoxazole. With sustained administration of acetyl sulfisoxazole, blood concentrations approximate those of sulfisoxazole. Following a single 4 gram dose of acetyl sulfisoxazole to healthy volunteers, maximum plasma concentrations of sulfisoxazole ranged from 122 to 282 mcg/mL (mean, 181 mcg/mL) for the pediatric suspension and occurred between 2 and 6 hours postadministration of sulfisoxazole, in a pharmacokinetic study .
Properties of SULFISOXAZOLE ACETYL (200 MG)
| Melting point: | 193-194° |
| Boiling point: | 538.1±60.0 °C(Predicted) |
| Density | 1.3541 (rough estimate) |
| refractive index | 1.6320 (estimate) |
| pka | -0.17±0.10(Predicted) |
| form | Solid |
| color | White to off-white |
| Water Solubility | 80mg/L(37 ºC) |
Safety information for SULFISOXAZOLE ACETYL (200 MG)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P362:Take off contaminated clothing and wash before reuse. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for SULFISOXAZOLE ACETYL (200 MG)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
![N-[(4-Aminophenyl)sulfonyl]-N-(3,4-dimethyl-5-isoxazolyl)acetamide CAS 80-74-0](https://img.chemicalbook.in//Content/image/CP5.jpg) N-[(4-Aminophenyl)sulfonyl]-N-(3,4-dimethyl-5-isoxazolyl)acetamide CAS 80-74-0View Details
N-[(4-Aminophenyl)sulfonyl]-N-(3,4-dimethyl-5-isoxazolyl)acetamide CAS 80-74-0View Details
80-74-0 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1