SULFENTRAZONE
- CAS NO.:122836-35-5
- Empirical Formula: C11H10Cl2F2N4O3S
- Molecular Weight: 387.19
- MDL number: MFCD00869633
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
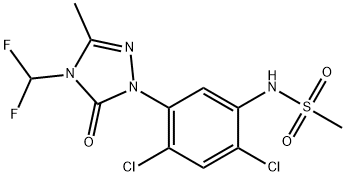
What is SULFENTRAZONE?
Description
Solubility. In water at 25 ?C: 100 mg/L at pH 6,
800 mg/mL at pH 7, 1,600 mg/mL at pH 7.5; soluble to
some extent in acetone and other polar organic solvents
Pka. 6.56
Stability. Stable
The Uses of SULFENTRAZONE
Herbicide.
The Uses of SULFENTRAZONE
Sulfentrazone is an herbicide used for controlling sedges in turfgrass.
Definition
ChEBI: Sulfentrazone is a member of the class of triazoles that is 5-oxo-1,2,4-triazole which is substituted at positions 1, 3, and 4 by 2,4-dichloro-5-[(methylsulfonyl)amino]phenyl, methyl, and difluoromethyl groups, respectively. A protoporphyrinogen oxidase inhibitor, it is used as a herbicide to control broad-leaved weeds in soya and tobacco crops. Not approved for use within the European Union. It has a role as an EC 1.3.3.4 (protoporphyrinogen oxidase) inhibitor, a herbicide and an agrochemical. It is a sulfonamide, a dichlorobenzene, an organofluorine compound and a member of triazoles.
Trade name
AUTHORITY® Sulfentrazone; CANOPY XL®; COVER®; F6285®; FMC® 97285; GAUNTLET®; SPARTAN®; SULFENTRAZONE® (F6285) 4F; SULFENTRAZONE® (F6285) 75DF
Metabolic pathway
When 14C-sulfentrazone is applied to coffee senna and sicklepod through the roots, 83% of the parent compound remains in coffee senna leaf tissue after 9 h exposure and in contrast, sicklepod takes up relatively less sulfentrazone through the root and metabolizes sulfentrazone in the foliage more rapidly than coffee senna. The primary detoxification reaction appears to be oxidation of the methyl group on the triazolinone ring, resulting in the formation of the more polar hydroxymethyl derivative. The aniline analog is identified as a plant-specific metabolite. The tolerance of sicklepod to sulfentrazone is primarily due to a relatively high rate of metabolism of sulfentrazone compared with coffee senna. When 14C-sulfentrazone is administered orally to rats, goats, and hens in a daily diet, administered radioactivity is quantitatively excreted in the urine, feces, or hen excreta. In all of the species, unchanged sulfentrazone and two non-conjugated metabolites are found, which are 3-hydroxymethyl and carboxylic acid derivatives, the latter of which decomposes at high temperature or acidic pH to give the corresponding desmethyl analog of sulfentrazone. In rats, a minor reduction metabolite is detected which is tentatively characterized as the 2,3-dihydro-3-hydroxymethyl derivative.
Properties of SULFENTRAZONE
| Melting point: | 75-78° |
| Boiling point: | 468.2±55.0 °C(Predicted) |
| Density | 1.21 g/cm3 |
| storage temp. | 0-6°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 6.56(at 25℃) |
| form | Solid |
| color | Pale Brown to Beige |
| EPA Substance Registry System | Sulfentrazone (122836-35-5) |
Safety information for SULFENTRAZONE
Computed Descriptors for SULFENTRAZONE
SULFENTRAZONE manufacturer
Hemani Industries Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2-(2,4-DICHLORO-PHENYL)-5-METHYL-2,4-DIHYDRO-[1,2,4]TRIAZOL-3-ONE](https://img.chemicalbook.in/CAS/GIF/79604-49-2.gif)

You may like
-
 122836-35-5 Sulfentrazone 99%View Details
122836-35-5 Sulfentrazone 99%View Details
122836-35-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1