SULBENICILLIN
- CAS NO.:41744-40-5
- Empirical Formula: C16H18N2O7S2
- Molecular Weight: 414.45
- MDL number: MFCD00869408
- EINECS: 255-528-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
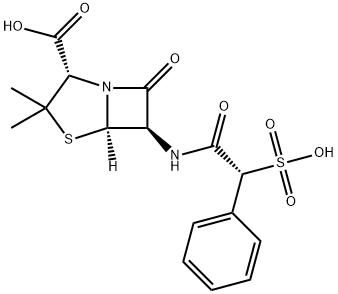
What is SULBENICILLIN?
Originator
Lillacillin,Takeda,Japan,1973
Definition
ChEBI: A penicillin antibiotic having a 6beta-[phenyl(sulfo)acetamido] side-chain.
Manufacturing Process
To a suspension of 1.08 parts by weight of 6-aminopenicillanic acid in 8 parts by volume of water is added 1.48 parts by weight of sodium bicarbonate. After the mixture is dissolved, a solution of 1.18 parts by weight of α-sulfophenylacetyl chloride in 10 parts by volume of diethylether is gradually added thereto. The mixture is stirred at a temperature in the neighborhood of 0°C for 1 hour. The aqueous layer is washed twice with 10 parts by volume of portions of ether and adjusted to pH 1.2 with cation exchange resin of polystyrene sulfonic acid type under constant cooling. Then the solution is washed twice with 15 parts by volume of portions of ethyl acetate, followed by extraction twice with 15 parts by volume of portions of n-butanol. The extracts are combined and washed twice with 15 parts by volume of portions of water and, then, extracted with an aqueous solution of sodium bicarbonate. The extract is adjusted to pH 6.5, washed with ether and lyophilized to give the sodium salt of α-sulfobenzylpenicillin. Yield is 1.2 parts by weight.
Therapeutic Function
Antibacterial
Antimicrobial activity
α-Sulfobenzylpenicillin, a semisynthetic penicillin supplied as the disodium salt. Its antimicrobial spectrum and pharmacokinetic pharmacokinetic behavior closely resemble those of carbenicillin. Following intravenous administration of 4 g, the mean plasma concentration at 1 h was approximately 160 mg/L, with a plasma elimination half-life of around 70 min. It is largely excreted in the urine, about 80% of the dose appearing in the first 24 h, less than 5% as the penicilloic acid. It has been noted that the penicilloic acid causes much stronger platelet aggregation than its parent. It is of very limited availability.
Properties of SULBENICILLIN
| Melting point: | 197 °C (decomp) |
| Density | 1?+-.0.1 g/cm3(Predicted) |
| pka | 0.28±0.50(Predicted) |
Safety information for SULBENICILLIN
Computed Descriptors for SULBENICILLIN
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1