Sucrose
Synonym(s):Sucrose;Sugar;α-D -Glc-(1→2)-β-D -Fru;α-D -Glucopyranosyl β-D -fructofuranoside;β-D -Fructofuranosyl-α-D -glucopyranoside
- CAS NO.:57-50-1
- Empirical Formula: C12H22O11
- Molecular Weight: 342.3
- MDL number: MFCD00006626
- EINECS: 200-334-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:46
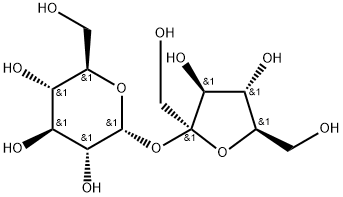
What is Sucrose?
Description
Sucrose is a disaccharide, or two-part molecule, formed by linking the monosaccharide sugars glucose and fructose. It is a nonreducing sugar, in contrast to the two sugars produced by the inversion reaction.
Chemical properties
White or almost white, crystalline powder, or lustrous, colourless or white or almost white crystals.
Chemical properties
Sucrose is a sugar obtained from sugar cane (Saccharum officinarum Linne' (Fam. Gramineae)), sugar beet (Beta vulgaris Linne' (Fam. Chenopodiaceae)), and other sources. It contains no added substances. Sucrose occurs as colorless crystals, as crystalline masses or blocks, or as a white crystalline powder; it is odorless and has a sweet taste.
History
Sucrose is the white granulated compound referred to as sugar. Sucrose is a disaccharide made of glucose and fructose. The main sources of sucrose for the production of commercial sugar are sugarcane and sugar beets. Sugarcane is a tall perennial grass of the genus Saccharum native to Southeast Asia and the South Pacifi c. It has been consumed by chewing the stalk in areas where it grows for thousands of years. Sugarcane spread to India where it was processed to extract crude sugar as early as 2,500 years ago. Persian invaders discovered sugar after invading India and the plant and sugar production spread into the Middle East around 600 c.e. Europeans were introduced to sugar around 1100 c.e. when the first crusaders returned with knowledge of the sweet spice and the Arab Empire spread into Spain.
The use of sugar beet to obtain sugar began when the German chemist Andreas Sigismund Marggraf (1709 1782) extracted sucrose from sugar beets using alcohol. The amount of sucrose obtained by Marggraf did not warrant commercial use of beets as a sucrose source. During the late 18th century, Franz Karl Archard (1753 1821), a student of Marggraf, selectively bred beets to increase the sucrose content to 5 6% and developed a commercial method to extract sucrose.
Sucrose is predominantly associated with the food industry, but it does have industrial uses in other areas. Sucrose fatty acid esters are a mixture of mono, di, and tri esters of sucrose with fatty acids. Th ese are use in cosmetics, shampoos, resins, inks, paper processing, and pesticides. Sucrose benzoate is used as an emulsifi er and in nail polishes. Sucrose has also been used in making glues and treating leather.
The Uses of Sucrose
Sucrose is a sweetener that is the disaccharide sucrose, consisting of one molecule of glucose and one molecule of fructose. It is obtained as cane or beet sugar. It has relatively constant solubility and is a universal sweetener because of its intense sweetness and solubility. It is available in various forms which include granulated, brown, and powdered. It is used in desserts, beverages, cakes, ice cream, icings, cereals, and baked goods. It is also termed beet sugar, cane sugar, and saccharose.
Background
A nonreducing disaccharide composed of glucose and fructose linked via their anomeric carbons. It is obtained commercially from sugarcane, sugar beet (beta vulgaris), and other plants and used extensively as a food and a sweetener.
Definition
ChEBI: Sucrose is a disaccharide formed by glucose and fructose units joined by an acetal oxygen bridge from hemiacetal of glucose to the hemiketal of the fructose.
Production Methods
Sucrose is obtained from the sugar cane plant, which contains 15–20% sucrose, and sugar beet, which contains 10–17% sucrose. Juice from these sources is heated to coagulate water-soluble proteins, which are removed by skimming. The resultant solution is then decolorized with an ion-exchange resin or charcoal and concentrated. Upon cooling, sucrose crystallizes out. The remaining solution is concentrated again and yields more sucrose, brown sugar, and molasses.
Definition
saccharose: A sugar comprising onemolecule of glucose linked to a fructosemolecule. It occurs widely inplants and is particularly abundant insugar cane and sugar beet (15–20%),from which it is extracted andrefied for table sugar. If heated to200°C, sucrose becomes caramel.
General Description
White odorless crystalline or powdery solid. Denser than water.
Air & Water Reactions
Water soluble. Sugar dust explosion is possibility.
Reactivity Profile
D(+)-Sucrose is a reducing agent. Can react explosively with oxidizing agents such as chlorates and perchlorates. Is hydrolyzed by dilute acids and by invertase (a yeast enzyme) . Chars rapidly and exothermically when mixed with concentrated sulfuric acid.
Hazard
Dental erosion. Questionable carcinogen.
Agricultural Uses
Sucrose is obtained from sugar beet, sugar cane and sweet sorghum. Sucrose, glucose and fructose all exhibit optical activity. When sucrose is hydrolyzed, the rotation changes from right to left. This is called inversion, and an equimolar mixture of glucose and fructose is called invert sugar. The enzyme invertase hydrolyzes sucrose to glucose and fructose.
Sugar occurs universally throughout the plant kingdom in fruits, seeds, flowers and roots.
Pharmaceutical Applications
Sucrose is widely used in oral pharmaceutical formulations.
Sucrose syrup, containing 50–67% w/w sucrose, is used in
tableting as a binding agent for wet granulation. In the powdered
form, sucrose serves as a dry binder (2–20% w/w) or as a bulking
agent and sweetener in chewable tablets and lozenges. Tablets
that contain large amounts of sucrose may harden to give poor
disintegration.
Sucrose syrups are used as tablet-coating agents at concentrations
between 50% and 67% w/w. With higher concentrations,
partial inversion of sucrose occurs, which makes sugar coating
difficult.
Sucrose syrups are also widely used as vehicles in oral liquiddosage
forms to enhance palatability or to increase viscosity.(4,5)
Sucrose has been used as a diluent in freeze-dried protein
products.
Sucrose is also widely used in foods and confectionery, and
therapeutically in sugar pastes that are used to promote wound
healing.
Safety Profile
Mildly toxic by ingestion. An experimental teratogen. Mutation data reported. Vigorous reaction with nitric acid or sulfuric acid (forms carbon monoxide and carbon dioxide). When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Sucrose is hydrolyzed in the small intestine by the enzyme sucrase to
yield dextrose and fructose, which are then absorbed. When
administered intravenously, sucrose is excreted unchanged in the
urine.
Although sucrose is very widely used in foods and pharmaceutical
formulations, sucrose consumption is a cause of concern and
should be monitored in patients with diabetes mellitus or other
metabolic sugar intolerance.
Sucrose is also considered to be more cariogenic than other
carbohydrates since it is more easily converted to dental plaque. For
this reason, its use in oral pharmaceutical formulations is declining.
Although sucrose has been associated with obesity, renal
damage, and a number of other diseases, conclusive evidence
linking sucrose intake with some diseases could not be established.(
13,14) It was, however, recommended that sucrose intake in
the diet should be reduced.
LD50 (mouse, IP): 14 g/kg
LD50 (rat, oral): 29.7 g/kg
Sucrose Application
Sucrose is used in pharmaceuticals as a flavor, as a preservative, as an antioxidant (in the form of invert sugar), as a demulcent, as substitute for glycerol, as granulation agent and excipient for tablets, as coating for tablets. In the plastics and cellulose industry, in rigid polyurethane foams, manufacture of ink and of transparent soaps.
Metabolism
Not Available
Storage
Sucrose has good stability at room temperature and at moderate
relative humidity. It absorbs up to 1% moisture, which is released
upon heating at 90°C. Sucrose caramelizes when heated to
temperatures above 160°C. Dilute sucrose solutions are liable to
fermentation by microorganisms but resist decomposition at higher
concentrations, e.g. above 60% w/w concentration. Aqueous
solutions may be sterilized by autoclaving or filtration.
When sucrose is used as a base for medicated confectionery, the
cooking process, at temperatures rising from 110 to 145℃, causes
some inversion to form dextrose and fructose (invert sugar). The
fructose imparts stickiness to confectionery but prevents cloudiness
due to graining. Inversion is accelerated particularly at temperatures
above 130°C and by the presence of acids.
Purification Methods
Crystallise D(+)-sucrose from water (solubility: 1g in 0.5mL H2O at 20o, 1g in 0.2mL in boiling H2O). It is soluble in EtOH (0.6%) and MeOH (1%). Sucrose diacetate hexaisobutyrate is purified by melting and, while molten, treated with NaHCO3 and charcoal, then filtered. [Beilstein 17/8 V 399.]
Incompatibilities
Powdered sucrose may be contaminated with traces of heavy metals, which can lead to incompatibility with active ingredients, e.g. ascorbic acid. Sucrose may also be contaminated with sulfite from the refining process. With high sulfite content, color changes can occur in sugar-coated tablets; for certain colors used in sugarcoating the maximum limit for sulfite content, calculated as sulfur, is 1 ppm. In the presence of dilute or concentrated acids, sucrose is hydrolyzed or inverted to dextrose and fructose (invert sugar). Sucrose may attack aluminum closures.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (injections; oral capsules, solutions, syrups, and tablets; topical preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Sucrose
| Melting point: | 185-187 °C (lit.) |
| Boiling point: | 397.76°C (rough estimate) |
| alpha | 67 º (c=26, in water 25 ºC) |
| Density | 1.5805 |
| refractive index | 66.5 ° (C=26, H2O) |
| Flash point: | 93.3°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 500 mg/mL |
| form | Liquid |
| pka | 12.7(at 25℃) |
| color | White |
| Odor | Odorless |
| PH | 5.0-7.0 (25℃, 1M in H2O) |
| PH Range | 5.5 - 7 at 342 g/l at 25 °C |
| optical activity | [α]25/D +66.3 to +66.8°(lit.) |
| Water Solubility | 1970 g/L (15 ºC) |
| λmax | λ: 260 nm Amax: 0.11 λ: 280 nm Amax: 0.08 |
| Merck | 14,8881 |
| BRN | 90825 |
| Exposure limits | ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 10 mg/m3; TWA 5 mg/m3 |
| Dielectric constant | 3.3(Ambient) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. Hydrolyzed by dilute acids and by invertase. |
| CAS DataBase Reference | 57-50-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Sucrose(57-50-1) |
| EPA Substance Registry System | Sucrose (57-50-1) |
Safety information for Sucrose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Sucrose
| InChIKey | CZMRCDWAGMRECN-UGDNZRGBSA-N |
Sucrose manufacturer
Akhil Healthcare Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 Sucrose, For analysis ACS CAS 57-50-1View Details
Sucrose, For analysis ACS CAS 57-50-1View Details
57-50-1 -
 Sucrose for cell culture CAS 57-50-1View Details
Sucrose for cell culture CAS 57-50-1View Details
57-50-1 -
 Sucrose for tissue culture CAS 57-50-1View Details
Sucrose for tissue culture CAS 57-50-1View Details
57-50-1 -
 Sucrose pure CAS 57-50-1View Details
Sucrose pure CAS 57-50-1View Details
57-50-1 -
 Sucrose IP (Non-Parenteral Use) CAS 57-50-1View Details
Sucrose IP (Non-Parenteral Use) CAS 57-50-1View Details
57-50-1 -
 Sucrose NF (USP) (Non-Parenteral Use) CAS 57-50-1View Details
Sucrose NF (USP) (Non-Parenteral Use) CAS 57-50-1View Details
57-50-1 -
 Sucrose CAS 57-50-1View Details
Sucrose CAS 57-50-1View Details
57-50-1 -
 Sucrose Extra Pure, Packaging Size: 500 Gm, Grade: LrView Details
Sucrose Extra Pure, Packaging Size: 500 Gm, Grade: LrView Details
57-50-1
