strontium phosphide
- CAS NO.:12504-13-1
- Empirical Formula: P2Sr3
- Molecular Weight: 324.81
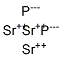
What is strontium phosphide?
Description
Strontium phosphide has the molecular formula of
Sr3P2 and the molecular weight of 324.8075 g/mol. Its
CAS number is 12504-13-1. It is a black crystalline material
with a melting point of 1205°C. Its density is
2.71 g/cm3. It readily reacts with water to form phosphine,
PH3, but is insoluble in ethanol.
It is easily prepared by reacting the metal with red
phosphorus at high temperature. The best way is to
sublime the P4 at 450°C in an inert gas stream and react
it with Sr metal at 650°C:
6Sr + 2P4 ? 2Sr3P4
Strontium phosphide is a solid which will evolve
flammable and toxic gases on contact with water. It
also can react vigorously with oxidizing materials. In
general, materials in this group are incompatible with
oxidizers such as atmospheric oxygen. They are
violently incompatible with acids, particularly oxidizing
acids.
General Description
strontium phosphide is a solid which will evolve flammable and toxic gasses on contact with water. strontium phosphide is a highly reactive material used as a laboratory reagent and in the manufacture of chemically reactive devices.
Air & Water Reactions
Decomposed by water or moisture in air evolving toxic phosphine which often ignites.
Reactivity Profile
strontium phosphide is a reducing agent. They slowly generate flammable or noxious gases in contact with water. Phosphides react quickly upon contact with moisture or acids to give the very toxic gas phosphine; phosphides also can react vigorously with oxidizing materials. In general, materials in this group are incompatible with oxidizers such as atmospheric oxygen. They are violently incompatible with acids, particularly oxidizing acids.
Health Hazard
Highly toxic: contact with water produces toxic gas, may be fatal if inhaled. Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable and toxic gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Containers may explode when heated. Runoff may create fire or explosion hazard.
Properties of strontium phosphide
| EPA Substance Registry System | Strontium phosphide (SrP) (12504-13-1) |
Safety information for strontium phosphide
Computed Descriptors for strontium phosphide
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran

You may like
-
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
 Cyclopentane carboxxylic acid 3400-45-1 99%View Details
Cyclopentane carboxxylic acid 3400-45-1 99%View Details
3400-45-1 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1 -
 Cyaclopentane carboxylic acid 99%View Details
Cyaclopentane carboxylic acid 99%View Details
3400-45-1 -
 2-Aminopyridine 504-29-0 99%View Details
2-Aminopyridine 504-29-0 99%View Details
504-29-0