Calcium hypophosphite
- CAS NO.:7789-79-9
- Empirical Formula: Ca(H2PO2)2
- Molecular Weight: 170.05
- MDL number: MFCD00867083
- EINECS: 232-190-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-10 18:54:13
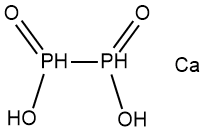
What is Calcium hypophosphite?
Description
Calcium hypophosphite has the molecular formula
of Ca(H2PO2)2 and the molecular weight of
170.0631 g/mol. This salt can be prepared by the
aqueous reaction of the carbonate or oxide with H3PO2:
CaO + 2H3PO2 ? Ca(H2PO2)2
The salt is soluble in water (20 g/100 ml at 20°C) so
that the solution must be evaporated to obtain crystals.
Ca(H2PO2)2 has the CAS number of 7789-79-9 and the
density of 1.59 g/cm3. It is composed of white square
monoclinic crystalswhich are water soluble. It is insoluble
in alcohol or ether.When heated to about 305°C, it disproportionates,
forming calcium phosphate and phosphine:
Ca(H2PO2)2 + heat ? CaHPO3 + PH3
Chemical properties
white monoclinic, prismatic crystal(s) or granular powder(s); used as a corrosion inhibitor and in medicine [MER06] [HAW93]
Physical properties
Calcium hypophosphite is relatively soluble in water,
and when added to a solution of sodium hypophosphite,
forms a double salt. Crystals were obtained by
slow evaporation of the solution to form dodecahedra
with well-developed {110} faces. The crystal structure
of CaNa(H2PO2)3 belongs to the cubic system with space
group, P213, Z = 4 and a = 9.721?. The structure
consists of two kinds of octahedral groups, one with
a calcium atom and the other with a sodium atom.
Each atom of the hypophosphite ions is coordinated to
each one of the calcium and sodium atoms to form the
octahedral. This structure is, in general, typical of the
salts of hypophosphorous acid.
Calcium hypophosphite is readily available commercially
since its main usage, both industrially and in
Research, has proven to be as a source of hypophosphite
anions to form other hypophosphites.
The Uses of Calcium hypophosphite
As corrosion inhibitor; in nickel plating. Pharmaceutic aid (retards oxidation of ferrous salts).
The Uses of Calcium hypophosphite
It is employed as dietary supplement. The product is used as a flame retardant and applied to chemical plating. Moreover, the product is used as a food additive and an animal nutrition agent, applied to the manufacture of pharmaceutical products, as well as antioxidant and analytical reagent.
Flammability and Explosibility
Highly flammable
Properties of Calcium hypophosphite
| Melting point: | decomposes [ALF93] |
| Boiling point: | 480℃[at 101 325 Pa] |
| Density | 2.243[at 20℃] |
| solubility | soluble in H2O; insoluble in ethanol |
| pka | 12.6[at 20 ℃] |
| form | white monoclinic crystals |
| color | white monoclinic crystals, crystalline |
| Water Solubility | Soluble in water. Practically insoluble in alcohol. Slightly soluble in glycerol |
| Sensitive | Hygroscopic |
| Merck | 14,1675 |
| CAS DataBase Reference | 7789-79-9(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium hypophosphite (7789-79-9) |
Safety information for Calcium hypophosphite
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Calcium hypophosphite
| InChIKey | XJPMAXXEWYLDCQ-UHFFFAOYSA-L |
Calcium hypophosphite manufacturer
Kronox Lab Sciences Pvt Ltd
Neemcco
Victory Chemicals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 CALCIUM HYPO PHOSPHITE 99%View Details
CALCIUM HYPO PHOSPHITE 99%View Details -
 Calcium hypophosphite 98%View Details
Calcium hypophosphite 98%View Details -
 Calcium hypophosphite 98%View Details
Calcium hypophosphite 98%View Details
7789-79-9 -
 7789-79-9 98%View Details
7789-79-9 98%View Details
7789-79-9 -
 Calcium hypophosphite 99%View Details
Calcium hypophosphite 99%View Details
7789-79-9 -
 7789-79-9 Calcium hypophosphite 98%View Details
7789-79-9 Calcium hypophosphite 98%View Details
7789-79-9 -
 Calcium hypophosphite, 99% CAS 7789-79-9View Details
Calcium hypophosphite, 99% CAS 7789-79-9View Details
7789-79-9 -
 Calcium phosphinate CAS 7789-79-9View Details
Calcium phosphinate CAS 7789-79-9View Details
7789-79-9