Stigmasterol
Synonym(s):3β-Hydroxy-24-ethyl-5,22-cholestadiene;5,22-Stigmastadien-3β-ol;Stigmasterin;Stigmasterin; Phytosterol; beta-Stigmasterol; D5-Stigmasterol; 110801
- CAS NO.:83-48-7
- Empirical Formula: C29H48O
- Molecular Weight: 412.7
- MDL number: MFCD00003630
- EINECS: 201-482-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
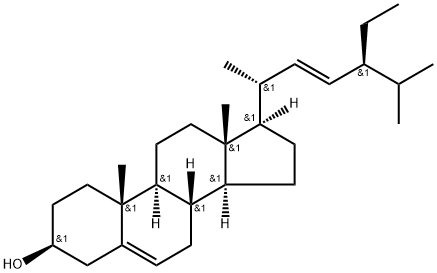
What is Stigmasterol?
Description
Stigmasterol is a natural plant sterol that can be isolated from certain seed oils and herbs, including those used for therapeutic purposes. Dietary consumption of phytosterols, including stigmasterol, may have beneficial health effects in adults, particularly against cancer and ulceration. Alternatively, phytosterols may contribute to inflammation and intestinal failure-
Chemical properties
white powder
The Uses of Stigmasterol
Stigmasterol has been used as a phytosterol compound to test its anti-proliferative property in breast cancer cell culture and its effect on liver X receptor-α (LXRA) activation. It may be used as an internal standard for the quantification of yeast cell wall sterols using gas chromatography–mass spectrometry (GC-MS) analysis, and in the preparation of lipid monolayers.
The Uses of Stigmasterol
Plant sterol, used as a precursor in the synthesis of progesterone.
What are the applications of Application
Stigmasterol is a phytosterol with chemical structure similar to cholesterol that exhibits anti-cancer, anti-pyretic, anti-inflammatory and immune-modulating effects
Definition
ChEBI: A 3beta-sterol that consists of 3beta-hydroxystigmastane having double bonds at the 5,6- and 22,23-positions.
General Description
Stigmasterol is a plant sterol that comprises an unsaturated bond between C22 and C23. It is found in foods like margarines and yogurts. Stigmasterol is synthesized from the mevalonate pathway and is present during development stages in plants.
Biochem/physiol Actions
Stigmasterol possesses anti-inflammatory anti-hypercholestrolemic, antitumor and antioxidant functionality. It plays a crucial role in the activation of plasma membrane H+-ATPase and cell proliferation. Variations in stigmasterol and its precursor are noticeable at both the seed and whole plant developmental stages. Stigmasterol may be involved in gravitropism and tolerance to abiotic stress.
storage
+4°C
Purification Methods
Stigmasterol is best purified via the tetrabromide-acetate. The impure sterol (3g) is acetylated with Ac2O (60mL) by refluxing for 1.5hour. The mixture is cooled at 20o for 1hour, and the crude acetate is collected. The acetate (3g) in Et2O (30mL) is then treated with Br2/AcOH (38mL, from 5g Br2 in 100mL AcOH), and after cooling at 6o overnight, the tetrabromoacetate is filtered off and washed with Et2O. After six recrystallisations from CHCl3/MeOH the tetrabromoacetate has m 194-196o. This product (1g) in AcOH (12mL) and Zn dust (1g) is refluxed for 1.5hours, filtered hot, diluted with H2O (30mL) and extracted with Et2O. The extract is washed with dilute aqueous sodium sulfite, then H2O, the extract is dried (Na2SO4) and the stigmasterol acetate (~550mg) is recrystallised (4x) from EtOH and twice from MeOH/CHCl3 (2:1) to give the acetate with m 139-148o. This acetate (400mg) is hydrolysed in boiling 10% alcoholic KOH (1mL) for 1hour. Then H2O (30mL) is added and the mixture is extracted with Et2O. The extract is washed with aqueous Na2CO3, then H2O, the solvent is distilled off and the residue is recrystallised (3x) from 95% EtOH to give ~110mg of pure stigmasterol. It is dried in a vacuum over P2O5 for 3hours at 90o. The purity is checked by NMR. The acetate crystallises from MeOH with m 145o, [] D 25 -56o (c 2, CHCl3). [Byerrum & Ball Biochemical Preparations 7 86 1959, Thornton et al. J Am Chem Soc 62 2006 1940, Colin et al. Anal Chem 51 1661 1979, Beilstein 6 IV 4170.]
Properties of Stigmasterol
| Melting point: | 165-167 °C (lit.) |
| Boiling point: | 472.07°C (rough estimate) |
| alpha | -50 º (c=2, CHCl3) |
| Density | 0.9639 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | -20°C |
| solubility | chloroform: soluble50 mg/ml |
| form | Powder |
| pka | 15.03±0.70(Predicted) |
| color | White |
| Water Solubility | insoluble |
| λmax | 226nm(MeOH)(lit.) |
| Merck | 14,8815 |
| BRN | 2568182 |
| CAS DataBase Reference | 83-48-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Stigmasterol(83-48-7) |
| EPA Substance Registry System | Stigmasterol (83-48-7) |
Safety information for Stigmasterol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Stigmasterol
Stigmasterol manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Stigmasterol 95% CAS 83-48-7View Details
Stigmasterol 95% CAS 83-48-7View Details
83-48-7 -
 Stigmasterol CAS 83-48-7View Details
Stigmasterol CAS 83-48-7View Details
83-48-7 -
 Stigmasterol extrapure CAS 83-48-7View Details
Stigmasterol extrapure CAS 83-48-7View Details
83-48-7 -
 Stigmasterol CAS 83-48-7View Details
Stigmasterol CAS 83-48-7View Details
83-48-7 -
 Stigmasterol CAS 83-48-7View Details
Stigmasterol CAS 83-48-7View Details
83-48-7 -
 Stigmasterol CAS 83-48-7View Details
Stigmasterol CAS 83-48-7View Details
83-48-7 -
 stigmasterol CAS 83-48-7View Details
stigmasterol CAS 83-48-7View Details
83-48-7 -
 Stigmasterol CAS 83-48-7View Details
Stigmasterol CAS 83-48-7View Details
83-48-7