Squalene
- CAS NO.:111-02-4
- Empirical Formula: C30H50
- Molecular Weight: 410.72
- MDL number: MFCD00008912
- EINECS: 203-826-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 14:26:18
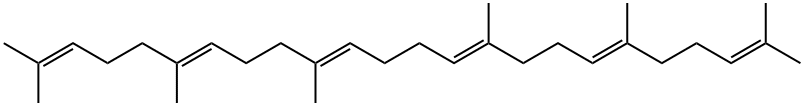
What is Squalene?
Description
Squalene is a natural unsaturated hydrocarbon that plays an important role in human health. It is an isoprenoid: an oligomer that consists of six isoprene units.
Squalene is an intermediate in the biosynthesis of all animal and plant biochemicals that have steroidal structures, including cholesterol and numerous human hormones. Its first isolation from fish liver oils is often attributed to Scottish chemist Isidor (later Ian) Morris Heilbron and coauthors in 1926; but Heilbron cites a 1906 article by Japanese chemist Mitsumaru Tsujimoto1 as the first report of the isolation of squalene.
Heilbron and colleagues worked out the structure of squalene in 1929. But perhaps the most important scientific work on squalene was the discovery of its biochemical pathway to cholesterol. In 1953, Robert G. Langdon and Konrad Bloch* at the University of Chicago used carbon-14 labeling to follow the route from acetate to squalene and then on to cholesterol. For their pioneering work on cholesterol and fatty acid metabolism, Bloch and Feodor Lynen at the Max Planck Institute for Cellular Chemistry (Munich) received the 1964 Nobel Prize in Physiology or Medicine.
Squalene is found in plant sources such as olive, wheat germ, and rice bran oils; but it is most abundant in fish liver oils, especially those harvested from sharks. Squalene is valuable as an adjuvant in vaccine formulations and (along with its hydrogenation product squalane) as an emollient in cosmetics. Its desirability has, almost predictably, resulted in widespread overfishing of sharks worldwide.
Efforts are under way to find other commercially feasible sources of squalene. Amyris (Emeryville, CA) makes it from products of sugar fermentation; and it can be extracted from olive oil–refining byproducts. The lip cosmetics company Axiology published an impassioned plea to save sharks by developing additional squalene sources.
1. According to Yojiro Tsuzuki at the Science University of Tokyo, Tsujimoto was the “father of lipid chemistry in Japan”; and his report “appears to be the first discovery of any hydrocarbon in the animal kingdom.”
Chemical properties
clear colourless to faint yellow oil
The Uses of Squalene
Squalene is a natural triterpene that plays an important role in the synthesis of cholesterol, steroid hormones, and vitamin D in the human body. Squalene is commonly used as a biochemical precursor i n the preparation of steroids. Squalene is also a natural moisturizer with low acute toxicity and is not significant human skin irritants or sensitizers.
The Uses of Squalene
cosmetic and pharmaceutical excipient
The Uses of Squalene
Bactericide; intermediate in manufacture of pharmaceuticals, organic coloring materials, rubber chemicals, aromatics and surface active agents.
What are the applications of Application
Squalene is a biosynthetic precursor for all steroids
Background
Squalene is originally obtained from shark liver oil. It is a natural 30-carbon isoprenoid compound and intermediate metabolite in the synthesis of cholesterol. It is not susceptible to lipid peroxidation and provides skin protection. It is ubiquitously distributed in human tissues where it is transported in serum generally in association with very low density lipoproteins. Squalene is investigated as an adjunctive cancer therapy.
Definition
ChEBI: A triterpene consisting of 2,6,10,15,19,23-hexamethyltetracosane having six double bonds at the 2-, 6-, 10-, 14-, 18- and 22-positions with (all-E)-configuration.
Production Methods
Squalene is extracted from shark liver or synthesized from hexaphenyl-1,4-butanediyldiphosphonium dibromide and 6,10-dimethyl-5,9-undecadien-2-one (geranylacetone, industrial intermediate in the vitamin E synthesis).
General Description
Clear, slightly yellow liquid with a faint odor. Density 0.858 g / cm3.
Air & Water Reactions
May become discolored on exposure to air. Insoluble in water.
Reactivity Profile
(all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene is incompatible with strong oxidizing agents. .
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition (all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene emits toxic fumes of carbon monoxide and carbon dioxide.
Fire Hazard
(all-E)-2,6,10,15,19,23-Hexamethyl-2,6,10,14,18,22-tetracosahexaene is probably combustible.
Flammability and Explosibility
Non flammable
Biochem/physiol Actions
Squalene is a biosynthetic precursor to all steroids. It acts as a cytoprotective agent to normal cells exposed to carcinogens and antitumor agents. Squalene helps in equalizing the blood cholesterol levels. It increases the production of HDL (high density lipoprotein) and the excretion of LDL (low density lipoprotein). This helps in reducing the risk of heart disease and protects the less stable body fats from oxidation. It is also used in treating hypercholesterolemia. Squalene is known to improve the efficiency of cholesterol lowering drugs. It also serves as an antioxidant by preventing the effects of free radical. It protects skin from drying and other environmental conditions such as oxidation, ultraviolet rays and pollutants. Squalene is known to promote wound healing.
Metabolism
Not Available
Properties of Squalene
| Melting point: | −75 °C(lit.) |
| Boiling point: | 285 °C25 mm Hg(lit.) |
| Density | 0.858 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 25℃ |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | DMSO : 16.67 mg/mL (40.59 mM; Need ultrasonic)H2O : < 0.1 mg/mL (insoluble) |
| appearance | pale yellow oil |
| form | liquid |
| color | light yellow |
| Odor | oil, faint odor |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| Merck | 14,8768 |
| BRN | 1728919 |
| CAS DataBase Reference | 111-02-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl-, (all-E)-(111-02-4) |
| EPA Substance Registry System | Squalene (111-02-4) |
Safety information for Squalene
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H304:Aspiration hazard |
Computed Descriptors for Squalene
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




![SQUALENE, [1,5,9,14,20,24-14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB6707635.gif)
You may like
-
 111-02-4 Trans-Squalene 98%View Details
111-02-4 Trans-Squalene 98%View Details
111-02-4 -
 Squalene CAS 111-02-4View Details
Squalene CAS 111-02-4View Details
111-02-4 -
 Squalene CAS 111-02-4View Details
Squalene CAS 111-02-4View Details
111-02-4 -
 Squalene CAS 111-02-4View Details
Squalene CAS 111-02-4View Details
111-02-4 -
 Squalene CAS 111-02-4View Details
Squalene CAS 111-02-4View Details
111-02-4 -
 Squalene CAS 111-02-4View Details
Squalene CAS 111-02-4View Details
111-02-4 -
 Squalene CAS 111-02-4View Details
Squalene CAS 111-02-4View Details
111-02-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
