Sodium hyaluronate
Synonym(s):Poly(β-glucuronic acid-[1→3]-β-N-acetylglucosamine-[1→4]), alternating;Sodium hyaluronate
- CAS NO.:9067-32-7
- Empirical Formula: C14H22NNaO11
- Molecular Weight: 403.31
- MDL number: MFCD00875848
- EINECS: 618-620-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-25 18:09:02
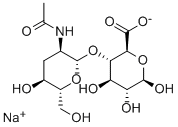
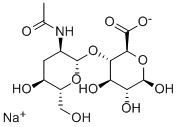
What is Sodium hyaluronate?
Chemical properties
The PhEur 6.3 describes sodium hyaluronate as the sodium salt of hyaluronic acid, a glycosaminoglycan consisting of D-glucuronic acid and N-acetyl-D-glucosamine disaccharide units. Sodium hyaluronate occurs as white to off-white powder or granules. It is very hygroscopic.
The Uses of Sodium hyaluronate
sodium hyaluronate is used as a humectant to increase moisture in the skin, it may also serve as an emulsifier. Sodium hyaluronate is capable of binding 1,800 times its own weight in water. It is the sodium salt of hyaluronic acid and is the most commonly used form.
What are the applications of Application
Hyaluronic Acid, Sodium Salt is a natural high-viscosity mucopolysaccharide with alternating β1,3-glucuronidic and β1,4-glucosaminidic bonds
Definition
Sodium Hyaluronate is a polysaccharide of linear and repeating structural units of the disaccharide D-glucuronic acid and N-acetylD-glucosamine. Hyaluronic acid in human body usually present as polymers with several million molecular weight, and the structural difference by the animal species has not been confirmed.
Production Methods
Sodium hyaluronate occurs naturally in vitreous humor, serum, chicken combs, shark skin, and whale cartilage; it is usually extracted and purified from chicken combs. It may also be manufactured by fermentation of selected Streptococcus zooepidemicus bacterial strains; sodium hyaluronate is removed from the fermentation medium by filtration and purified by ultrafiltration. It is then precipitated with an organic solvent and dried.
What are the applications of Application
Sodium Hyaluronic is is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. Sodium hyaluronate is widely used as follows:
antifungal agent.
prevents thromboembolic complications.
aldosterone antagonist used as an adjunct in the management of chronic heart failure.
suitable as substrate for hyaluronidase.
Surgical aid (ophthalmological).
brand name
Equron [Veterinary] (Fort Dodge Animal Health); Legend (Bayer Animal Health); Synacid [Veterinary] (Schering-Plough Animal Health).
Pharmaceutical Applications
Sodium hyaluronate is the predominant form of hyaluronic acid at
physiological pH. The name hyaluronan is used when the
polysaccharide is mentioned in general terms, and in the literature
the terms hyaluronic acid and sodium hyaluronate are used
interchangeably.
Hyaluronan is used therapeutically to treat osteoarthritis in the
knee, and is an effective treatment for arthritic pain. Crosslinked
hyaluronan gels are used as drug delivery systems.
Hyaluronan is the most common negatively charged glycosaminoglycan
in the human vitreous humor, and is known to interact
with polymeric and liposomal DNA complexes, where hyaluronan
solutions have been shown to decrease the cellular uptake of
complexes.(4) This is useful for enhancing the availability and
retention time of drugs administered to the eye. It is immunoneutral,
which makes it useful for the attachment of biomaterials for use in
tissue engineering and drug delivery systems; it also has important
applications in the fields of vascosurgery and vascosupplementation.
Biochem/physiol Actions
High molecular mass polymer composed of repeating dimeric units of glucuronic acid and N-acetyl glucosamine which forms the core of complex proteoglycan aggregates found in extracellular matrix.
Safety
Sodium hyaluronate is used in cosmetics and in topical, parenteral,
and ophthalmic pharmaceutical formulations. It is generally
regarded as a relatively nontoxic and nonirritant material. Sodium
hyaluronate has been reported to be an experimental teratogen.
LD50 (mouse, IP): 1.5 g/kg
LD50 (rabbit, IP): 1.82 g/kg
LD50 (rat, IP): 1.77 g/kg
storage
Sodium hyaluronate should be stored in a cool, dry place in tightly sealed containers. The powder is stable for 3 years if stored in unopened containers.
Regulatory Status
Included in the FDA Inactive Ingredients Database (topical gel preparation).
Properties of Sodium hyaluronate
| Melting point: | >209°C (dec.) |
| alpha | D25 -74° (c = 0.25 in water): Rapport et al., J. Am. Chem. Soc. 73, 2416 (1951) |
| storage temp. | -20°C |
| solubility | H2O: 5 mg/mL, clear, colorless |
| form | Powder |
| color | White to cream |
| PH | pH(2g/l,25℃) : 5.5~7.5 |
| Water Solubility | SOLUBLE |
| Merck | 13,4776 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 9067-32-7(CAS DataBase Reference) |
Safety information for Sodium hyaluronate
Computed Descriptors for Sodium hyaluronate
| InChIKey | MAKUBRYLFHZREJ-JWBQXVCJSA-M |
| SMILES | [C@@H]1(O[C@H]2[C@H](O)[C@H]([C@H](O)O[C@@H]2C(=O)[O-])O)O[C@H](CO)[C@@H](O)C[C@H]1NC(=O)C.[Na+] |&1:0,2,3,5,6,9,15,18,21,r| |
Sodium hyaluronate manufacturer
Fido Pharma
Godrej Industries Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 Hyaluronic acid sodium salt, <i>Streptococcus equi</i> CAS 9067-32-7View Details
Hyaluronic acid sodium salt, <i>Streptococcus equi</i> CAS 9067-32-7View Details
9067-32-7 -
 Hyaluronic Acid Sodium Salt - Type 4 (40-50 kD) extrapure CAS 9067-32-7View Details
Hyaluronic Acid Sodium Salt - Type 4 (40-50 kD) extrapure CAS 9067-32-7View Details
9067-32-7 -
 Hyaluronic Acid Sodium Salt - Type 3 (80-100 kD) extrapure CAS 9067-32-7View Details
Hyaluronic Acid Sodium Salt - Type 3 (80-100 kD) extrapure CAS 9067-32-7View Details
9067-32-7 -
 Hyaluronic acid sodium salt 95% CAS 9067-32-7View Details
Hyaluronic acid sodium salt 95% CAS 9067-32-7View Details
9067-32-7 -
 Hyaluronic acid sodium salt, from streptococcus, mol wt 1,750,000-2,000,000 CAS 9067-32-7View Details
Hyaluronic acid sodium salt, from streptococcus, mol wt 1,750,000-2,000,000 CAS 9067-32-7View Details
9067-32-7 -
 Hyaluronic acid sodium salt, from bovine vitreous humor CAS 9067-32-7View Details
Hyaluronic acid sodium salt, from bovine vitreous humor CAS 9067-32-7View Details
9067-32-7 -
 Hyaluronic Acid Sodium Salt - Type 5 (8-15 kD) extrapure CAS 9067-32-7View Details
Hyaluronic Acid Sodium Salt - Type 5 (8-15 kD) extrapure CAS 9067-32-7View Details
9067-32-7 -
 Hyaluronic Acid Sodium Salt - Type 2 (1000-2000 kD) extrapure CAS 9067-32-7View Details
Hyaluronic Acid Sodium Salt - Type 2 (1000-2000 kD) extrapure CAS 9067-32-7View Details
9067-32-7