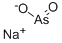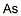sodium arsenate
- CAS NO.:15120-17-9
- Empirical Formula: AsH2NaO3
- Molecular Weight: 147.92
- MDL number: MFCD01941460
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
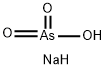
What is sodium arsenate?
Chemical properties
White crystalline, odorless solids.
Safety Profile
Confirmed human carcinogen. A poison. Mutation data reported. See also ARSENIC COMPOUNDS. When heated to decomposition it emits toxic fumes of arsenic.
Potential Exposure
(dibasic): Sodium arsenate is used in dyeing and printing; making other arsenates; as a germicide; in dyeing with turkey-red oil.
Shipping
UN1685 Sodium arsenate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Sodium arsenate reacts with moisture, steam, and water; forming flammable and toxic arsenic hydride. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Sodium arsenate is a weak oxidizing agent that may react with reducing agents, including hydrides. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, chemically active metals. Arsine, a very deadly gas, can be released in the presence of acid, acid mists; or hydrogen gas. Attacks many metals (such as aluminum, iron, and zinc) in presence of moisture producing arsenic and arsine fumes
Waste Disposal
Dissolve in minimum quantity of concentrated, reagent hydrochloric acid. Filter if necessary. Dilute with water until white precipitate forms. Add just enough 6M HCl to redissolve. Saturate with hydrogen sulfide. Filter, wash the precipitate; dry, package, and ship to the supplier.
Properties of sodium arsenate
| color | Needle-like fibrous hygroscopic crystals |
| EPA Substance Registry System | Arsenenic acid, sodium salt (15120-17-9) |
Safety information for sodium arsenate
Computed Descriptors for sodium arsenate
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1