SIS3
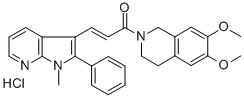
Synonym(s):(2E)-1-(6,7-Dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)-3-(1-methyl-2-phenyl-1H-pyrrolo[2,3-b]pyridin-3-yl)-propenone hydrochloride
- CAS NO.:521984-48-5
- Empirical Formula: C28H27N3O3HCl
- Molecular Weight: 0
- MDL number: MFCD09265258
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is SIS3?
The Uses of SIS3
Sugar insensitive 3 (SIS3) has been used as smad3?inhibitor in various cells.
What are the applications of Application
SIS3 hydrochloride is a specific inhibitor of Smad3 phosphorylation
Definition
ChEBI: SIS3 is a hydrochloride resulting from the reaction of SIS3 free base with 1 mol eq. of hydrogen chloride. It has a role as a Smad3 inhibitor. It contains a SIS3 free base(1+).
Biological Activity
sis3 is an inhibitor of smad3.the receptor-associated smads, such as smad2 and smad3, directly interact with activated tgf-receptor type i. smads form heteromeric complexes with smad4, which is a common mediator for all smad pathways.
Biochem/physiol Actions
Sugar insensitive 3 (SIS3) promotes drug-induced apoptosis and hinders the function of adenosine triphosphate (ATP)-binding cassette (ABC) transporter (ABCB1) and ABCG2. It resensitizes ABCB1 and ABCG2 overexpressed in cancerous cells, which contributes to chemotherapeutics.
in vitro
in the reporter assay, it was found that the increased luciferase activity of p3tp-lux could be abrogated by the sis3 treatment in a dosedependent manner. immunoprecipitation revealed sis3 attenuated the tgf-1-induced phosphorylation of smad3 and interaction of smad3 with smad4. whereas, sis3 did not affect the phosphorylation of smad2. in addition, it was found that sis3 attenuated the effects of tgf-1 by reducing the transcriptional activity. sis3 could also inhibit the myofibroblast differentiation of fibroblasts by tgf-1. moreover, sis3 diminished the constitutive phosphorylation of smad3 completely [1].
in vivo
animal study showed that, in tie2-cre;loxp-egfp mice, ages could induce endomt. immunoprecipitation and western blotting showed that smad3 could be activated by ages but was inhibited by sis3 in both mmecs and in stz-induced diabetic nephropathy. furthermore, confocal microscopy and real-time pcr showed that sis3 could abrogate endomt, reduce renal fibrosis, as well as retard nephropathy progression [2].
storage
Store at -20°C
References
[1] jinnin m et al. characterization of sis3, a novel specific inhibitor of smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. mol pharmacol. 2006 feb;69(2):597-607.
[2] li j et al. blockade of endothelial-mesenchymal transition by a smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. diabetes.2010 oct;59(10):2612-24.
Properties of SIS3
| Melting point: | >161°C (dec.) |
| storage temp. | 2-8°C |
| solubility | H2O: <2mg/mL |
| form | powder |
| color | yellow |
Safety information for SIS3
Computed Descriptors for SIS3
| InChIKey | CDKIEBFIMCSCBB-CALJPSDSSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 SIS3 CAS 521984-48-5View Details
SIS3 CAS 521984-48-5View Details
521984-48-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8
![1H-Pyrrolo[2,3-b]pyridine,1,3-dimethyl-](https://img.chemicalbook.in/CAS/GIF/464180-72-1.gif)
![1H-Pyrrolo[2,3-b]pyridine, 2,3-dimethyl-](https://img.chemicalbook.in/CAS/GIF/10299-69-1.gif)