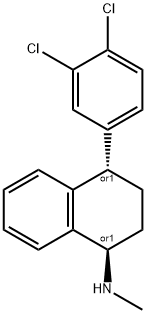
Sertraline
- CAS NO.:79617-96-2
- Empirical Formula: C17H17Cl2N
- Molecular Weight: 306.23
- MDL number: MFCD00865372
- EINECS: 605-001-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
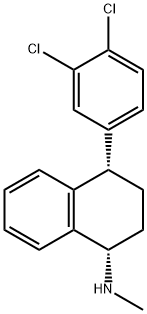
What is Sertraline?
Absorption
Following once-daily administration of 50 to 200 mg for two weeks, the mean peak plasma concentrations (Cmax) of sertraline occurred between 4.5 to 8.4 hours after administration, and measured at 20 to 55 μg/L. Steady-state concentrations are reached after 1 week following once-daily administration, and vary greatly depending on the patient. Bioavailability has been estimated to be above 44%. The area under the curve in healthy volunteers after a 100mg dose of sertraline was 456 μg × h/mL in one study.
Effects of food on absorption
The effects of food on the bioavailability of the sertraline tablet and oral concentrate were studied in subjects given a single dose with and without food. For the tablet, AUC was slightly increased when sertraline was administered with food, the Cmax was 25% greater, and the time to peak plasma concentration was shortened by about 2.5 hours. For the oral concentrate preparation of sertraline, peak concentration was prolonged by approximately 1 hour with the ingestion of food.
Toxicity
The LD50 of sertraline is >2000 mg/kg in rats according to the FDA label. One other references indicates an oral LD50 of in mice and rats of 419 - 548 mg/kg and 1327 - 1591mg/kg, respectively.
The most common signs and symptoms associated with a non-fatal sertraline overdose are somnolence, vomiting, tachycardia, nausea, dizziness, agitation, and tremor. No cases of fatal overdose with only sertraline have been reported. Most fatal cases are associated with the ingestion of sertraline with other drugs. Consequences of a sertraline overdose may include serotonin syndrome, hypertension, hypotension, syncope, stupor, coma, bradycardia, bundle branch block, QT-prolongation, torsade de pointes, delirium, hallucinations, and pancreatitis.
Description
Sertraline, best known by the brand name Zoloft (Pfizer, New York City), is a venerable antidepressant that was approved by the US Food and Drug Administration in 1991. Its mode of action, common among antidepressants, is as a selective serotonin reuptake inhibitor. The active ingredient in most of its formulations is its hydrochloride1.
Sertraline continues to be a frequently prescribed antidepressant (≈40 million prescriptions in 2019). But its success has come with an environmental cost: This year, a review by South African scientists Lawrence Mzukisi Madikizela* and Somandla Ncubeb cited dozens of drugs, including sertraline, that have been detected in marine organisms and seafood. This finding is a major concern for ocean life because of the aquatic toxicity statements shown in the hazard information table.
1. CAS Reg. No. 79559-97-0.
Originator
Lustral,Pfizer,UK
The Uses of Sertraline
cis-Sertraline is an isomer of Sertraline. Sertraline is a selective serotonin reuptake inhibitor. It is used as an antidepressant.
The Uses of Sertraline
Antidepressant;5-HT uptake inhibitor
Background
Sertraline is a popular antidepressant medication commonly known as a selective serotonin reuptake inhibitor (SSRI), and is similar to drugs such as Citalopram and Fluoxetine. Despite marked structural differences between compounds in this drug class, SSRIs exert similar pharmacological effects.
Several weeks of therapy with sertraline may be required before beneficial effects are noticed. Sertraline displays enhanced safety or tolerability than other classes of antidepressants, which frequently cause high levels of drowsiness, dizziness, blurred vision, and other undesirable effects.
Indications
Sertraline is indicated for the management of major depressive disorder (MDD), post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), panic disorder (PD), premenstrual dysphoric disorder (PMDD), and social anxiety disorder (SAD). Common off-label uses for sertraline include the prevention of post stroke depression, generalized anxiety disorder (GAD), fibromyalgia, premature ejaculation, migraine prophylaxis, diabetic neuropathy, and neurocardiogenic syncope.
Definition
ChEBI: A member of the class of tetralins that is tetralin which is substituted at positions 1 and 4 by a methylamino and a 3,4-dichlorophenyl group, respectively (the S,S diastereoisomer). A selective serotonin-reuptake inhibito (SSRI), it is administered orally as the hydrochloride salt as an antidepressant for the treatment of depression, obsessive-compulsive disorder, panic disorder and post-traumatic stress disorder.
brand name
Zoloft (Pfizer).
Therapeutic Function
Antidepressant
Biological Functions
Sertraline (Zoloft) has an elimination half-life of 25 hours and can be administered once a day, usually in the morning to avoid insomnia. Sertraline undergoes extensive hepatic metabolism, and doses must be reduced in patients with liver disease. Sertraline may produce more gastrointestinal side effects, such as nausea and diarrhea, than does fluoxetine and is generally thought to be less activating than fluoxetine. It is highly bound to serum proteins (98%) and may alter plasma protein binding of other medications.A 14-day washout period is recommended before starting a MAOI. Sertraline is a weak inhibitor of cytochrome P450 2D6. Intensive therapeutic drug monitoring is indicated when combining sertraline with drugs metabolized by this route that have a narrow therapeutic index, such as the TCAs and the type 1C antiarrhythmics propafenone, encainide, and flecainide.
General Description
Inspection of sertraline (Zoloft) (1S,4S) reveals the pharmacophorefor SERT inhibition. The Cl substituents alsopredict tropism for a 5-HT system. The depicted stereochemistryis important for activity.
Mechanism of action
Sertraline is a potent and selective inhibitor of the neuronal reuptake 5-HT transporter. In vitro binding studies suggest that sertraline has a substantially higher selectivity for inhibiting 5-HT reuptake than other SSRIs or TCAs, including clomipramine. It has only weak effects on neuronal uptake of NE and dopamine. Its mechanism of action is common to the SSRIs. Sertraline is very selective, lacking affinity for other neuroreceptors at therapeutic concentrations.
Pharmacokinetics
Sertraline improves or relieves the symptoms of depression, OCD, post-traumatic stress disorder, obsessive-compulsive disorder, panic disorder, and premenstrual dysphoric disorder via the inhibition of serotonin reuptake. Clinical studies have shown that it improves cognition in depressed patients. It has less sedative, anticholinergic, and cardiovascular effects than the tricyclic antidepressant drugs because it does not exert significant anticholinergic, antihistamine, or adrenergic (alpha1, alpha2, beta) blocking activity. The onset of action and beneficial effects are usually noticed after 4-6 weeks, for reasons that are not fully understood and currently under investigation.
Pharmacokinetics
Sertraline appears to be slowly but well absorbed from the GI tract following oral administration. The oral bioavailability of sertraline in humans ranges from 20 to 36%, suggesting extensive first-pass metabolism to its N-desmethylated metabolite. Food enhances its oral absorption decreasing the time to achieve peak plasma concentrations from approximately 8 to 6 hours. Following multiple dosing, steady-state plasma sertraline concentrations are proportional and linearly related to dose (half-life: single dose, 24 hours; multiple dose, 24 hours). N-desmethylsertraline, sertraline's principal metabolite, exhibits dose-dependent pharmacokinetics. Sertraline and N-desmethylsertraline are distributed into breast milk. Although in elderly patients the elimination half-life is increased to approximately 36 hours, this effect does not appear to be clinically important and does not warrant dosing alterations. Sertraline is primarily metabolized by CYP3A4 N-demethylation in the intestine and liver to its principal metabolite N-desmethylsertraline and several other metabolites. N-desmethylsertraline is approximately 5- to 10 times less potent as an inhibitor of 5-HT reuptake than sertraline. Sertraline and N-desmethylsertraline undergo further metabolism via oxidative deamination and ring hydroxylation and glucuronide conjugation. N-desmethylsertraline has an elimination half-life approximately 2.5 times that of sertraline. Following oral administration, sertraline and its conjugated metabolites are excreted in both urine and feces, and unmetabolized sertraline accounts for less than 5% of oral dose. Plasma clearance of sertraline was approximately 40% lower in geriatric patients. The elimination half-life of sertraline in patients with hepatic disease was prolonged to a mean of 52 hours, compared with 22 hours in individuals without hepatic disease.
Clinical Use
SSRI:
Antidepressant
Post-traumatic stress disorder
Obsessive compulsive disorder
Drug interactions
Sertraline is not a potent inhibitor of CYP3A4, and because CYP2D6 metabolism is a minor pathway for
sertraline, drug–drug interactions with these isoforms is unlikely to be of clinical importance. Sertraline is
metabolized by more than one CYP isoform in parallel; therefore, drug interactions or genetic polymorphisms
are unlikely to cause clinically significant drug interaction via CYP isoform inhibition. Caution is advised,
however, when coadministering sertraline with potential object drugs, especially those with narrow
therapeutic indices in elderly patients. For example, sertraline has been shown to reduce the clearance of
desipramine and imipramine as a result of CYP2D6 inhibition.
Because sertraline is highly protein bound, patients receiving it concurrently with any highly protein-bound
drug should be observed for potential adverse effects associated with combined therapy.
Metabolism
Sertraline is heavily metabolized in the liver and has one major active metabolite. It undergoes N-demethylation to form N-desmethylsertraline, which is much less potent in its pharmacological activity than sertraline. In addition to N-demethylation, sertraline metabolism involves N-hydroxylation, oxidative deamination, and finally, glucuronidation. The metabolism of sertraline is mainly catalyzed by CYP3A4 and CYP2B6, with some activity accounted for by CYP2C19 and CYP2D6.
Metabolism
Sertraline undergoes extensive first-pass metabolism in the liver. The main pathway is demethylation to inactive N-desmethylsertraline, a process that appears to involve multiple cytochrome P450 isoenzymes; further metabolism and glucuronide conjugation occurs. Sertraline is excreted in about equal amounts in the urine and faeces, mainly as metabolites.
Properties of Sertraline
| Boiling point: | 416.3±45.0 °C(Predicted) |
| Density | 1.25±0.1 g/cm3(Predicted) |
| solubility | 3.8 g/l |
| appearance | white crystals or powder |
| pka | pKa 9.48±0.04(H2O
I > 0)(Approximate) |
| Water Solubility | <0.1g/L(room temperature) |
| InChI | InChI=1S/C17H17Cl2N/c1-20-17-9-7-12(13-4-2-3-5-14(13)17)11-6-8-15(18)16(19)10-11/h2-6,8,10,12,17,20H,7,9H2,1H3/t12-,17-/m0/s1 |
| CAS DataBase Reference | 79617-96-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Sertraline(79617-96-2) |
| EPA Substance Registry System | Sertraline (79617-96-2) |
Safety information for Sertraline
Computed Descriptors for Sertraline
| InChIKey | VGKDLMBJGBXTGI-SJCJKPOMSA-N |
| SMILES | [C@@H]1(NC)C2=C(C=CC=C2)[C@H](C2=CC=C(Cl)C(Cl)=C2)CC1 |
Sertraline manufacturer
Chemeca Drugs Private Limited (Vegesna Laboratories Pvt Ltd)
HRV Global Life Sciences
Sibram Pharmaceutical
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 79617-96-2 SERTRALINE 20% DC GRANULES 98%View Details
79617-96-2 SERTRALINE 20% DC GRANULES 98%View Details
79617-96-2 -
 79617-96-2 98%View Details
79617-96-2 98%View Details
79617-96-2 -
 Sertraline 98%View Details
Sertraline 98%View Details
79617-96-2 -
 79617-96-2 Sertraline 99%View Details
79617-96-2 Sertraline 99%View Details
79617-96-2 -
 79617-96-2 98%View Details
79617-96-2 98%View Details
79617-96-2 -
 Sertraline 99%View Details
Sertraline 99%View Details -
 79617-96-2 Sertraline 99%View Details
79617-96-2 Sertraline 99%View Details
79617-96-2 -
 79617-96-2 99%View Details
79617-96-2 99%View Details
79617-96-2