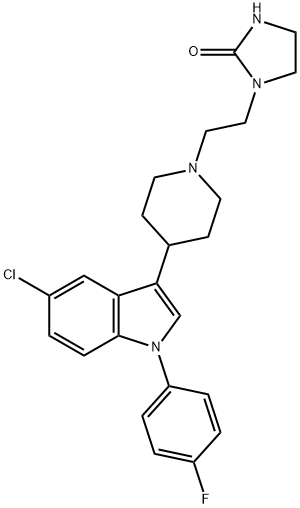
SERTINDOLE
Synonym(s):1-[2-[4-[5-Chloro-1-(4-fluorophenyl)-1h-indol-3-yl]-1-piperidinyl]ethyl]-2-imidazolidinone
- CAS NO.:106516-24-9
- Empirical Formula: C24H26ClFN4O
- Molecular Weight: 440.94
- MDL number: MFCD00867749
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is SERTINDOLE?
Absorption
Orally available.
Description
Serdolect was launched in the UK for acute and chronic schizophrenia and schizoaffective psychoses. It is synthetically available in three steps from 5chloro-l- (4-fluorophenyl)-l H-indole. Sertindole is selective for the limbic areas of the brain and has a high affinity for the serotinin 5-HT2 receptor where it behaves as an antagonist. It has weak affinity for the α1-adrenergic receptor and no affinity for dopamine D2 receptors. It is as effective as haloperidol but without the extrapyramidal symptoms. This may be a consequence of the fact that it expresses the c-fos protein in a manner different for haloperidol but similar to clozapine. It is non-sedating, long lasting (several days after a single dose) and has an atypical mode of action.
Description
Sertindole is an atypical antipsychotic that binds to dopamine D2 receptors and the serotonin (5-HT) receptor subtypes 5-HT1D, 5-HT2A, and 5-HT2C (Kds = 2.7, 20, 0.14, and 6 nM, respectively). It also binds to histamine H1 and α1- and α2-adrenergic receptors (Kds = 320, 3.9, and 190 nM, respectively). In vivo, sertindole (10 mg/kg) increases extracellular dopamine, acetylcholine (ACh), and glutamate levels in the medial prefrontal cortex in conscious rats. Sertindole (2.5 mg/kg) reverses ketamine-induced impairments in the extradimensional shift stage of the attentional set-shifting task (ASST) in rats. It reverses phencyclidine (PCP)-induced selective reversal learning deficits in rats and subchronic PCP-induced deficits in the novel object recognition task in mice. Sertindole (0.02-0.32 mg/kg) also prevents accuracy deficits and anticipatory over-responding in the 5-choice serial reaction time task (5CSRTT) induced by the NMDA receptor antagonist (R)-CPP in rats. Formulations containing sertindole have been used in the treatment of schizophrenia.
Chemical properties
Off-White to Pale Yellow Solid
Originator
Lundbeck (Denmark)
The Uses of SERTINDOLE
Antipsychotic used in the treatment of schizophrenia.
The Uses of SERTINDOLE
antipsychotic;Dopamine D2/Serotonin 5-HT2 receptor antagonist
What are the applications of Application
Sertindole is a SR-2 (5-HT2) and D2DR (D2 receptor) antagonist
Background
Sertindole, a neuroleptic, is one of the newer antipsychotic medications available. Serdolect is developed by the Danish pharmaceutical company H. Lundbeck. It is a phenylindole derivative used in the treatment of schizophrenia. It was first marketed in 1996 in several European countries before being withdrawn two years later because of numerous cardiac adverse effects. It has once again been approved and should soon be available on the French and Australian market.
Indications
Used in the treatment of schizophrenia.
Definition
ChEBI: A phenylindole that is 1H-indole which is substituted on the nitrogen by a p-chlorophenyl group, at position 5 by chlorine, and at position 3 by a piperidin-4-yl group, which is itself substituted on the nitrogen by a 2-(2-o oimidazolidin-1-yl)ethyl group.
brand name
SerLect (Abbott).
Pharmacokinetics
Sertindole is an atypical antipsychotic at least as effective as haloperidol and risperidone in the treatment of neuroleptic-responsive schizophrenia. Sertindole improves negative symptoms, and is also effective for the treatment of neuroleptic-resistant schizophrenia. Sertindole is generally well tolerated and is associated with a low rate of extrapyramidal symptoms (EPS).
Side Effects
Sertindole is an indole-containing compound that behaves as a high-affinity serotonin 5-HT2 receptor antagonist, with weak affinity for adrenergic α1 receptors and almost no affinity for dopaminergic D2 receptors. It is about as effective as haloperidol in the treatment of acute and chronic schizophrenia, but with much lower incidence of extrapyramidal side effects. Sertindole is relatively nonsedating, and its effects are long lasting (several days).
Metabolism
Hepatic. Sertindole is metabolized by cytochrome P450 isoenzymes CYP 2D6 and CYP 3A4.
Properties of SERTINDOLE
| Melting point: | 95-100°C |
| Boiling point: | 592.1±50.0 °C(Predicted) |
| Density | 1.36 |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO: >10mg/mL |
| form | solid |
| pka | 14.53±0.20(Predicted) |
| color | off-white |
| Merck | 14,8466 |
Safety information for SERTINDOLE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for SERTINDOLE
| InChIKey | GZKLJWGUPQBVJQ-UHFFFAOYSA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Sertindole CAS 106516-24-9View Details
Sertindole CAS 106516-24-9View Details
106516-24-9 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1