Sertaconazole nitrate
Synonym(s):1-[2-[(7-Chlorobenzo[b]thien-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole nitrate
- CAS NO.:99592-32-2
- Empirical Formula: C20H15Cl3N2OS
- Molecular Weight: 437.77
- MDL number: MFCD00868881
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-29 18:06:31
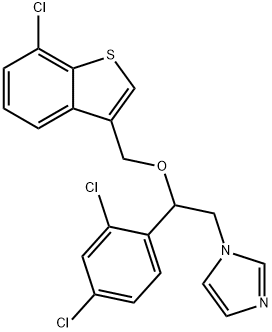
What is Sertaconazole nitrate?
Absorption
Bioavailability is negligible.
Description
Sertaconazole has been developed and launched for the treatment of dermatological fungal infections by Ferrer Internacional S. A. Mylan received FDA approval for sertaconazole nitrate cream for the treatment of athlete's foot (tinea pedis) at the end of 2003.
The Uses of Sertaconazole nitrate
An imidazole antifungal agent, inhibits the synthesis of ergosterol, an essential cell wall component of fungi.
Background
Sertaconazole nitrate is an antifungal medication of the imidazole class. It is available in topical formulations for the treatment of skin infections such as athlete's foot.
Indications
For the topical treatment of interdigital tinea pedis in immunocompetent patients 12 years of age and older, caused by Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum.
Definition
ChEBI: 1-{2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}imidazole is a member of the class of imidazoles that carries a 2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl group at position 1. It is a dichlorobenzene, an ether, a member of imidazoles and a member of 1-benzothiophenes.
Indications
Topical treatment of mycoses of the skin induced or sustained by fungi such as yeasts and dermatophytes. New formulations for the treatment of vaginal mycoses are in development.
Antimicrobial activity
Sertaconazole is a rather new broad-spectrum imidazole antimycotic with activity against almost all species of pathogenic fungi. It also has excellent activity against pathogenic yeasts.
Pharmacokinetics
Sertaconazole is an imidazole/triazole type antifungal agent. Sertaconazole is a highly selective inhibitor of fungal cytochrome P-450 sterol C-14 α-demethylation via the inhibition of the enzyme cytochrome P450 14α-demethylase. This enzyme converts lanosterol to ergosterol, and is required in fungal cell wall synthesis. The subsequent loss of normal sterols correlates with the accumulation of 14 α-methyl sterols in fungi and may be partly responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition. Sertaconazole exhibits in vitro activity against Cryptococcus neoformans and Candida spp. Fungistatic activity has also been demonstrated in normal and immunocompromised animal models for systemic and intracranial fungal infections due to Cryptococcus neoformans and for systemic infections due to Candida albicans.
Synthesis
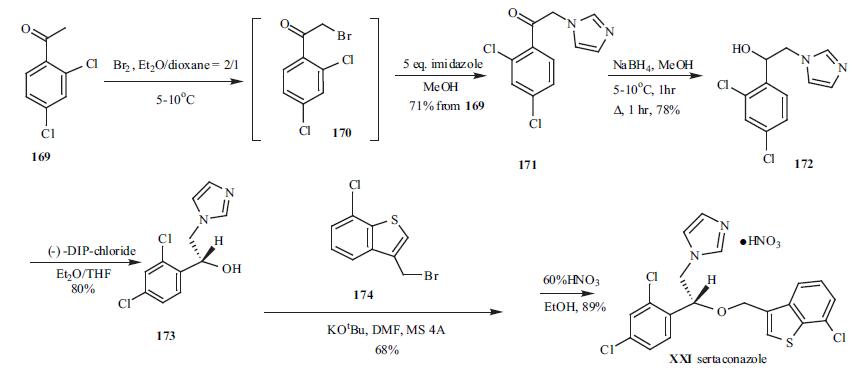
2,4-Dichloro acetophenone 169 was brominated at low temperature to give bromide intermediate 170, which was used without isolation. To the same pot, five-fold excess of imidazole was added to give imidazolylacetophenone 171 in 71% yield from 169. Sodium borohydride was employed to reduce ketone 171 to alcohol 172 in 78% yield. Racemic alcohol 172 was resolved with (-)-DIP-chloride to give its corresponding chiral R-alcohol 173 in 80% yield. Compound 173 was then alkylated with 3-bromomethyl-7-chlorobenzo[b]thiophene (174) in dry DMF in the presence of potassium t-butoxide to give the alkylation product in 68% yield. Finally, 60% nitric acid was used to make sertaconazole mononitrate (XXI) in 89% yield.
Metabolism
Not Available
Solubility in organics
Fairly soluble in ethanol (1.7 %), chloroform (1.5 %); slightly soluble in acetone (0.95 %); very slightly soluble in noctanol (0.069 %). Practically insoluble in water (< 0.01 %).
Properties of Sertaconazole nitrate
| Melting point: | 146-147° |
| Boiling point: | 614.1±55.0 °C(Predicted) |
| Density | 1.43±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 6.68±0.12(Predicted) |
| color | White to Pale Yellow |
| CAS DataBase Reference | 99592-32-2(CAS DataBase Reference) |
Safety information for Sertaconazole nitrate
Computed Descriptors for Sertaconazole nitrate
Sertaconazole nitrate manufacturer
HRV Global Life Sciences
Bioaltus Laboratories Pvt Ltd
Aspen Biopharma Labs Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 99592-32-2 SERTACONAZOLEView Details
99592-32-2 SERTACONAZOLEView Details
99592-32-2 -
 99592-32-2 99%View Details
99592-32-2 99%View Details
99592-32-2 -
 99592-32-2 Sertaconazole 98%View Details
99592-32-2 Sertaconazole 98%View Details
99592-32-2 -
 Sertaconazole 99%View Details
Sertaconazole 99%View Details -
 Sertaconazole 99592-32-2 99%View Details
Sertaconazole 99592-32-2 99%View Details
99592-32-2 -
 99592-32-2 98%View Details
99592-32-2 98%View Details
99592-32-2 -
 Sertaconazole 98%View Details
Sertaconazole 98%View Details
99592-32-2 -
 Sertaconazole 98%View Details
Sertaconazole 98%View Details
99592-32-2