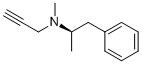
Selegiline hydrochloride
Synonym(s):(R)-(−)-N,α-Dimethyl-N-(2-propynyl)phenethylamine hydrochloride;R-(−)-Deprenyl hydrochloride;R(−)-N-α-Dimethyl-N-2-propynyl-benzeneethanamine hydrochloride;Selegiline hydrochloride
- CAS NO.:14611-52-0
- Empirical Formula: C13H18ClN
- Molecular Weight: 223.74
- MDL number: MFCD00069299
- EINECS: 604-508-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-05 20:55:42
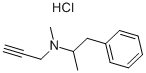
What is Selegiline hydrochloride?
Description
Deprenyl (14611-52-0) is a potent inhibitor of monoamine oxidase B (MAO- B) which has been used for the treatment of Parkinson’s disease.1,2 Displays neuroprotective effects rescuing nigral dopaminergic neurons after systemic MPTP treatment.3 Rescues PC12 cells from trophic withdrawal-induced apoptosis.4 Glyceraldehyde-3-phosphate dehydrogenase has been found to be the putative target responsible for its neuroprotective effects.5
Chemical properties
Crystalline Solid
The Uses of Selegiline hydrochloride
Selegiline hydrochloride is used to alleviate the symptonms of Parkinsons disease
The Uses of Selegiline hydrochloride
antibacterial
The Uses of Selegiline hydrochloride
Antidepressant, Antiparkinsonian
What are the applications of Application
R(?)-Deprenyl hydrochloride is a neuroprotective agent and inhibitor of MAO-B
brand name
Eldepryl (Somerset); Zelapar (Valeant).
Biological Activity
Selective inhibitor of monoamine oxidase B (MAO-B).
Clinical Use
Monoamine-oxidase-B inhibitor:
Treatment of Parkinson’s disease
Veterinary Drugs and Treatments
Selegiline is approved for use in dogs for the treatment of Cushing’s disease and for Canine Cognitive Dysfunction (so-called “old dog dementia”). Its use for Cushing’s disease is somewhat controversial as clinical studies evaluating its efficacy have shown disappointing results. In humans, selegiline’s primary indication is for the adjunctive treatment of Parkinson’s disease.
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: hyperpyrexia and CNS toxicity reported
with pethidine - avoid; avoid with opioid analgesics.
Antidepressants: avoid with citalopram and
escitalopram; increased risk of hypertension and
CNS excitation with fluvoxamine, sertraline or
venlafaxine, do not start selegiline until 1 week after
stopping them, avoid for 2 weeks after stopping
selegiline; increased risk of hypertension and CNS
excitation with paroxetine, do not start selegiline
until 2 weeks after stopping paroxetine, avoid for 2
weeks after stopping selegiline avoid concomitant
use with other MAOIs and moclobemide (can
lead to hypertensive crisis) - allow at least 14 days
before starting a MAOI; avoid concomitant use with
fluoxetine, allow 5 weeks between stopping fluoxetine
and starting selegiline; allow 14 days between
stopping selegiline and starting fluoxetine; increased
CNS toxicity with tricyclics and vortioxetine.
Oestrogens and progestogens: concentration of
selegiline increased - avoid.
Sympathomimetics: concomitant use is not
recommended; risk of hypertensive crisis with
dopamine.
Metabolism
Extensive first-pass metabolism in the liver to produce
at least 5 metabolites, including desmethylselegiline
(norselegiline), N-methylamfetamine, and amfetamine.
Plasma concentrations of selegiline metabolites are greatly
reduced after doses of the oral lyophilisate preparation,
the majority of which undergoes absorption through the
buccal mucosa.
Selegiline is excreted as metabolites mainly in the urine
and about 15% appears in the faeces.
References
Gerlach et al. (1992), The molecular pharmacology of L-deprenyl; Eur. J. Pharmacol., 226 97 Tetrud and Langston (1989), The effect of deprenyl (selegiline) on the natural history of Parkinson’s disease; Science, 245 519 Tatton and Greenwood (1991), Rescue of dying neurons: a new action of deprenyl in MPTP parkinsonism; J. Neurosci Res., 30 666 Tatton et al. (1994), (-)-Deprenyl reduces PC12 cell apoptosis by inducing new protein synthesis; J. Neurochem, 63 1572 Kargten et al. (1998), Glyceraldehyde-3-phosphate dehydrogenase, the putative target of the antiapoptotic compounds CGP 3466 and R-(-)-deprenyl; J. Biol. Chem., 273 5821
Properties of Selegiline hydrochloride
| Melting point: | 141-142°C |
| alpha | D25 -10.8° (c = 6.48 in water) |
| storage temp. | 2-8°C |
| solubility | H2O: >10 mg/mL |
| form | solid |
| color | white |
| optical activity | [α]25/D 10.8°, c = 6.48 in H2O(lit.) |
| Water Solubility | Soluble in water at 100mM |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 14611-52-0(CAS DataBase Reference) |
Safety information for Selegiline hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
Computed Descriptors for Selegiline hydrochloride
Abamectin manufacturer
Embio Limited
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![SELEGILINE HYDROCHLORIDE, [N-METHYL-3H],SELEGILINE HYDROCHLORIDE, [N-METHYL-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB2235561.gif)


You may like
-
 Selegiline hydrochloride 99%View Details
Selegiline hydrochloride 99%View Details -
 14611-52-0 98%View Details
14611-52-0 98%View Details
14611-52-0 -
 Selegiline HCl 98% (HPLC) CAS 14611-52-0View Details
Selegiline HCl 98% (HPLC) CAS 14611-52-0View Details
14611-52-0 -
 Selegiline Hydrochloride CAS 14611-52-0View Details
Selegiline Hydrochloride CAS 14611-52-0View Details
14611-52-0 -
 Selegiline hydrochloride CAS 14611-52-0View Details
Selegiline hydrochloride CAS 14611-52-0View Details
14611-52-0 -
 R-(−)-Deprenyl hydrochloride CAS 14611-52-0View Details
R-(−)-Deprenyl hydrochloride CAS 14611-52-0View Details
14611-52-0 -
 Selegiline hydrochloride CAS 14611-52-0View Details
Selegiline hydrochloride CAS 14611-52-0View Details
14611-52-0 -
 Selegiline hydrochloride CAS 14611-52-0View Details
Selegiline hydrochloride CAS 14611-52-0View Details
14611-52-0