SEDANOLIDE
- CAS NO.:6415-59-4
- Empirical Formula: C12H18O2
- Molecular Weight: 194.27
- MDL number: MFCD00171327
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
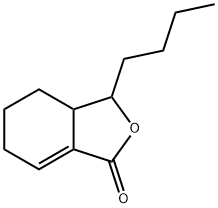
What is SEDANOLIDE?
The Uses of SEDANOLIDE
Sedanolide is a natural phthalide isolates from seed oil of Umbelliferae family. It induces the expression of glutathione S-transferase and reduces chemical induced carcinogenesis in mice.
What are the applications of Application
Sedanolide is an inducer of GST and inhibitor of Cox-1, Cox-2, Topo I, Topo II, and carcinogenesis
Definition
ChEBI: Sedanolide is a member of 2-benzofurans.
Biological Activity
sedanolide is a natural compound produced in edible umbelliferous plants, such as celery seed oil [1].in hepg2 and caco-2 cells, treatment with sedanolide (7-500 μm) for 24h showed no effect on cell viability. in hepg2 cells cultured in sedanolide-free medium, sedanolide (500 μm) treatment for 72h decreased cell viability. pretreatment with sedanolide (100 μm) for 24 h and exposement to either h2o2 or tbooh did not exhibit statistically significant difference in viability from controls. in hepg2 following 24-h incubation with 500 μm sedanolide, a significant increase in dna strand breaks was observed. sedanolide did not modulate h2o2- and tbooh-induced dna damage. sedanolide was relatively nontoxic to cells in culture [1]. sedanolide (sn) possesses antioxidant effects. in human liver cancer (j5) cells, treatment with sedanolide suppressed j5 cell viability by inducing autophagy. sedanolide decreased protein expression levels of phosphoinositide 3-kinase (pi3k)-i, mammalian target of rapamycin (mtor) and akt and increased pi3k-iii, lc3-ii and beclin-1 protein levels. sedanolide increased the cytosolic phosphorylation of inhibitor of kappa b (iκb) and nuclear p65 and the dna-binding activity of nf-κb. sedanolide induced j5 cell autophagy by regulating pi3k, p53 and nf-κb autophagy-associated signaling pathways in j5 cells [2]. sedanolide (100 μg/ml) inhibited cyclooxygenases-1 and -2 at 250 pg/ml and blocked topoisomerase-i and-ii activity [3].
References
[1] woods j a, jewell c, o'brien n m. sedanolide, a natural phthalide from celery seed oil: effect on hydrogen peroxide and tert-butyl hydroperoxide-induced toxicity in hepg2 and caco-2 human cell lines[j]. in vitro & molecular toxicology: a journal of basic and applied research, 2001, 14(3): 233-240.
[2] hsieh s l, chen c t, wang j j, et al. sedanolide induces autophagy through the pi3k, p53 and nf-κb signaling pathways in human liver cancer cells[j]. international journal of oncology, 2015, 47(6): 2240-2246
[3] momin r a, nair m g. antioxidant, cyclooxygenase and topoisomerase inhibitory compounds from apium graveolens linn. seeds[j]. phytomedicine, 2002, 9(4): 312-318.
Properties of SEDANOLIDE
| Melting point: | 30~31℃ |
| Boiling point: | 342.0±11.0 °C(Predicted) |
| Density | 1.03 |
| storage temp. | Store at -20°C, protect from light |
| solubility | insoluble in H2O; ≥52.8 mg/mL in EtOH; ≥6.65 mg/mL in DMSO |
| form | White to off-white solid. |
| color | White to off-white |
| Odor | at 100.00 %. herbal celery |
| CAS DataBase Reference | 6415-59-4(CAS DataBase Reference) |
Safety information for SEDANOLIDE
Computed Descriptors for SEDANOLIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran
![2-THIO(3-IODOBENZYL)-5-(1-PYRIDYL)-[1,3,4]-OXADIAZOLE](https://img.chemicalbook.in/StructureFile/ChemBookStructure7/GIF/CB2201711.gif)
![S-[N-(3-PHENYLPROPYL)(THIOCARBAMOYL)]-L-CYSTEINE](https://img.chemicalbook.in/CAS/GIF/137915-13-0.gif)
You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5