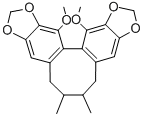
Schisandrin
Synonym(s):Schisandrin;Schizandrin;Wuweizi alcohol A;Wuweizichun A;(+)-Schizandrin
- CAS NO.:7432-28-2
- Empirical Formula: C24H32O7
- Molecular Weight: 432.51
- MDL number: MFCD00905761
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
What is Schisandrin?
Description
Schizandrin is a dibenzocyclooctadiene lignan and a major component of S. chinensis and has diverse biological activities. It induces cell cycle arrest at the G0/G1 phase and inhibits growth of T47D and MDA-MB-231 breast cancer cells when used at a concentration of 100 μM. Schizandrin (10 and 100 μM) prevents glutamate-induced cytotoxicity, inhibits production of nitric oxide (NO) and reactive oxygen species (ROS), and preserves the mitochondrial membrane potential in isolated rat cortical cells. It reduces apoptosis induced by cisplatin in HK-2 human kidney cells. In vivo, schizandrin (1 and 10 mg/kg, p.o.) reverses scopolamine-induced impairment of spatial memory and the passive avoidance response in rats. It enhances oxotremorine-induced tremors in mice. Schizandrin (10 mg/kg) reduces serum levels of IgE, IgG1, IL-4, and IFN-γ in an ovalbumin-sensitized mouse model of allergy.
The Uses of Schisandrin
Schizandrin may be used in oxidative stress-related cell signaling studies.
What are the applications of Application
Schizandrin is a natural compound that belongs to the group of schisandrins. Schizandrins are a class of compounds with biochemical properties and biological activities, such as anti-inflammatory, antioxidant, and anti-cancer effects. This compound has been shown to have pro-apoptotic properties in a model system. It has also demonstrated the ability to inhibit influenza A virus replication in vitro and protect mice against Listeria monocytogenes infection. It can be used as an inhibitor of gsh-px activity in myocardial infarcts.
Definition
ChEBI: Schizandrin is a tannin.
Biochem/physiol Actions
Schizandrin is a natural product with several biological properties involved with antioxidant and anti-inflammatory activities. It reduces the formation of ROS, inhibits the mitochondrial pathway of the apoptotic process and oxidative stress. Schizandrin has been reported to have hepatoprotective, antitumor, antiviral, and antiamnesic effects, and has neuroprotective activity against glutamate induced neurotoxicity.
Properties of Schisandrin
| Melting point: | 128.5°C |
| Boiling point: | 466.17°C (rough estimate) |
| Density | 1.1480 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥13mg/mL |
| form | powder |
| pka | 14.58±0.40(Predicted) |
| color | white to beige |
| optical activity | [α]/D 75 to 95°, c = 1 in methanol |
Safety information for Schisandrin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Schisandrin
| InChIKey | YEFOAORQXAOVJQ-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
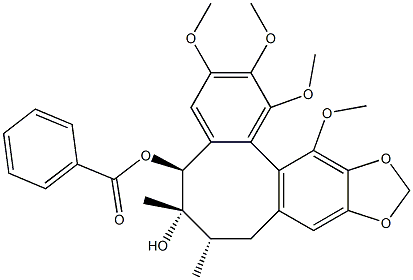
![(5S)-6α,7β-Dimethyl-1,2,3,11,12-pentamethoxy(5,6,7,8-tetrahydrodibenzo[a,c]cyclooctene)-5α,6β,10-triol 5-benzoate](https://img.chemicalbook.in/CAS/20150408/GIF/64917-83-5.gif)

You may like
-
 Schizandrin 98% (HPLC) CAS 7432-28-2View Details
Schizandrin 98% (HPLC) CAS 7432-28-2View Details
7432-28-2 -
 Schisandrol A CAS 7432-28-2View Details
Schisandrol A CAS 7432-28-2View Details
7432-28-2 -
 Schisandrin CAS 7432-28-2View Details
Schisandrin CAS 7432-28-2View Details
7432-28-2 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0