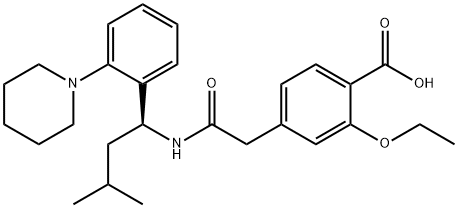
Saroglitazar
- CAS NO.:495399-09-2
- Empirical Formula: C25H29NO4S
- Molecular Weight: 439.57
- MDL number: MFCD28502034
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
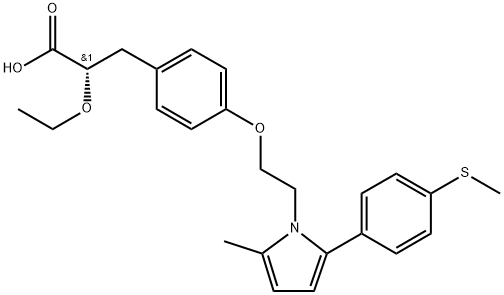
What is Saroglitazar?
Description
Saroglitazar is a dual agonist of PPARα and PPARγ (EC50s = 0.65 and 3,000 pM, respectively, in a transactivation assay in HepG2 cells). It decreases serum triglyceride, free fatty acid, and glucose levels in a db/db mouse model of diabetes when administered at doses ranging from 0.01 to 3 mg/kg per day for 12 days. It increases insulin sensitivity in an oral glucose challenge when administered at a dose of 1 mg/kg in db/db mice, as well as decreases LDL levels in hApoB100/hCETP mice and in hamsters fed a high-fat high-cholesterol diet. Saroglitazar (10 μM) reverses palmitic acid-induced decreases in the expression of superoxide dismutase 1 (SOD1), SOD2, glutathione peroxidase (GPX), and catalase and increases in TNF-α, IL-1β, and IL-6 expression in HepG2 cells. It decreases hepatic inflammation and steatosis in a mouse model of non-alcoholic steatohepatitis (NASH) induced by a choline-deficient high-fat diet when administered at a dose of 3 mg/kg and inhibits fibrosis in a mouse model of fibrosis induced by carbon tetrachloride.
Description
Saroglitazar (also known as ZYH1) was approved for use by the Drug Controller General of India for the treatment of diabetic dyslipidemia and hypertriglyceridemia that is not controlled by statin therapy. Saroglitazar is the first approved agent with dual PPAR agonist activity, with EC50s of 0.00065 and 3 nM, respectively, for the α- and γ-PPAR isoforms in HepG2 cells. The medicinal chemistry program has not been described in the scientific literature, but numerous substituents on the pyrrole ring are described in the saroglitazar patent. These substituted pyrroles were synthesized via ethanolamine condensation with substituted diketones and subsequent activation of the hydroxyethylpyrrole for phenolic etherification with (S)-2- ethoxy-3-(4-hydroxyphenyl)propanoic acid. The efficacy of saroglitazar was evaluated in db/db mice (genetically diabetic animals that lack the leptin receptor), wherein it produced a 17–55% reduction in serum triglycerides (TGs) after 12 days of administration of saroglitazar (0.01–3 mg/kg).
Originator
Cadila Healthcare Ltd. (India)
The Uses of Saroglitazar
Saroglitazar, is a drug for the treatment of diabetic dyslipidemia and hypertriglyceridemia with Type 2 diabetes mellitus not controlled by statin therapy. Its trade name is Lipaglyn. It is also a 1,2-Diarylpyrroles derivative, which can be used in the preparation of Nonsteroidal anti-inflammatory drugs (NSAIDs).
Definition
ChEBI: Saroglitazar is a monocarboxylic acid that is (2S)-2-ethoxy-3-(p-ethoxyphenyl)propanoic acid in which one of the methyl hydrogens of the p-ethoxy substituent has been replaced by the nitrogen of 2-methyl-5-[4-(methylthio)phenyl]-1H-pyrrole. An agonist at the subtypes alpha and gamma of the peroxisome proliferator-activated receptor (PPAR) with predominant PPARalpha activity, it is used in the treatment of type 2 diabetes. It has a role as a PPARgamma agonist, a hypoglycemic agent and a PPARalpha agonist. It is a member of pyrroles, a monocarboxylic acid, a methyl sulfide and an aromatic ether.
brand name
Lipaglyn
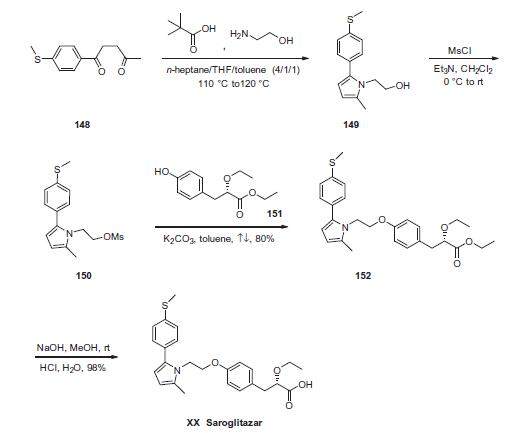
Synthesis
The sequence began with a Paal¨CKnorr pyrrole synthesis starting from commercial 1-[4-(methylthio)phenyl]pentane-1,4-dione (148). Subjection of this diketone to ethanolamine in warm pivalic acid furnished pyrrole 149, which was taken forward as the crude product. Next, the alcohol was mesylated to afford 150, which was used without further purification. Williamson ether conditions were employed to convert mesylate 150 to the corresponding aryl ether 152 through the use of commercial phenol 151 and anhydrous potassium carbonate. This reaction proceeded in 80% yield. Saponification of the terminal ethyl ester using sodium hydroxide followed by acidic pH adjustment ultimately delivered the carboxylic acid drug saroglitazar (XX) in 98% yield from 152.
Properties of Saroglitazar
| Boiling point: | 621.0±55.0 °C(Predicted) |
| Density | 1.15±0.1 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Gel |
| pka | 3.60±0.10(Predicted) |
| color | Red |
Safety information for Saroglitazar
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Saroglitazar
Saroglitazar manufacturer
Zydus Lifesciences Ltd.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 495399-09-2 Saroglitazar 98%View Details
495399-09-2 Saroglitazar 98%View Details
495399-09-2 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1