Salmeterol
- CAS NO.:89365-50-4
- Empirical Formula: C25H37NO4
- Molecular Weight: 415.57
- MDL number: MFCD00867037
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-29 15:43:00
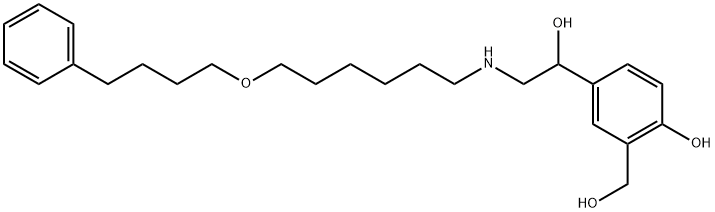
What is Salmeterol?
Absorption
In asthmatic patients, a 50μg dose of inhaled salmeterol powder reaches a Cmax of 47.897pg/mL, with a Tmax of 0.240h, and an AUC of 156.041pg/mL/h.
Toxicity
Patients experiencing an overdose have presented with metabolic acidosis, hyperlactatemia, anxiety, palpitations, chest pain, sinus tachycardia, ST depression, hypokalemia, hypophosphatemia. Though patients may also present with seizures, angina, hypertension or hypotension, arrhythmia, headache, tremor, muscle cramps, dry mouth, nausea, dizziness, fatigue, malaise, insomnia, and hyperglycemia. Patients should be given symptomatic and supportive treatment which may include intravenous fluids, potassium supplementation, a cardioselective beta-blocker, and cardiac monitoring.
Data regarding the LD50 of salmeterol is not readily available.
Description
Salmeterol is a long-acting β2- agonist for patients with chronic asthma, useful also in controlling persistent nocturnal asthma.
The Uses of Salmeterol
Salmeterol is a β2-Adrenergic agonist used for relief and control of asthma symptoms.
The Uses of Salmeterol
Bronchodilator.
What are the applications of Application
Salmeterol is a potent and selective β2-AR agonist
Indications
Salmeterol is indicated in the treatment of asthma with an inhaled corticosteroid, prevention of exercise induced bronchospasm, and the maintenance of airflow obstruction and prevention of exacerbations of chronic obstructive pulmonary disease.
Background
Salmeterol is a long-acting beta-2 adrenergic receptor agonist drug that is currently prescribed for the treatment of asthma and chronic obstructive pulmonary disease COPD. It has a longer duration of action than the short-acting beta-2 adrenergic receptor agonist, salbutamol. Salmeterol was first described in the literature in 1988. Salmeterol's structure is similar to salbutamol's with an aralkyloxy-alkyl substitution on the amine.
Salmeterol was granted FDA approval on 4 February 1994.
Definition
ChEBI: A phenol having a hydroxymethyl group at C-2 and a 1-hydroxy-2-{[6-(4-phenylbutoxy)hexyl]amino}ethyl group at C-4; derivative of phenylethanolamine.
General Description
Salmeterol has an N-phenylbutoxyhexyl substituent incombination with a β-OH group and a salicyl phenyl ring foroptimal direct-acting β2-receptor selectivity and potency. Ithas a potency similar to that of ISO. This drug associateswith the β2-receptor slowly resulting in slow onset of actionand dissociates from the receptor at an even slower rate. It is resistant to both MAO and COMT and highly lipophilic(log P=3.88). It is thus very long acting (12 hours), aneffect also attributed to the highly lipophilic phenylalkylsubstituent on the nitrogen atom, which is believed to interactwith a site outside but adjacent to the active site.
Hazard
A poison by inhalation.
Biological Activity
Potent and selective β 2 -adrenoceptor agonist (EC 50 = 5.3 nM); bronchodilator. Unlike other β 2 agonists, binds to exo-site domain of β 2 receptors, producing a slow onset of action and prolonged activation. Also available as part of the β -Adrenoceptor Agonist Tocriset™ .
Mechanism of action
A β2-agonist with a slow onset and extended duration of action is salmeterol. Salmeterol has the same phenyl ring substitution R3 as albuterol but also an unusually long and lipophilic group R1 on the nitrogen. The octanol–water partition coefficient, log P, for salmeterol is 3.88, compared with 0.66 for albuterol. Salmeterol is approximately 50-fold more selective than albuterol for the β2-receptor. Substantial evidence indicates that its long duration of action results from a specific binding interaction (“anchoring”) of the phenyl group at the end of the extended lipophilic side chain with a specific region of the β2-receptor, affording salmeterol a unique binding mechanism.
Pharmacokinetics
Salmeterol is a long acting beta-2 adrenergic receptor agonist that binds to both the active and exo sites of the beta-2 adrenergic receptor. Salmeterol has a longer duration of action than other beta-2 agonists like salbutamol. Patients should be counselled regarding the risks of long acting beta agonist (LABA) monotherapy, hypokalemia, hypoglycemia, and not to take this drug with another LABA.
Pharmacokinetics
Salmeterol is 94 to 98% bound to human plasma proteins, both albumin and α1-acid glycoprotein, in vitro over the concentration range of 8 to 7722μg of base/L, much higher concentrations than those achieved following therapeutic doses of this drug. When salmeterol is inhaled, plasma concentrations of the drug often cannot be detected, even 30 minutes after administration of therapeutic doses. Larger inhaled doses give approximately proportionally increased blood concentrations. Plasma salmeterol concentrations of 0.1 to 0.2 and 1 to 2 μg/L were attained in healthy volunteers about 5 to 15 minutes after inhalation of single doses of 50 and 400μg, respectively. These concentrations were most probably related to the drug passing rapidly through the alveolar-capillary interface and probably into mucosal and submucosal vessels. In patients who inhaled salmeterol 50μg twice a day for 10 months, a second Cmax of 0.07 to 0.2 μg/L occurred 45 to 90 minutes after inhalation, probably due to gastrointestinal absorption of the swallowed drug (most of the dose delivered by a metered-dose inhaler is swallowed). Oral administration of 1mg of radiolabelled [14C]salmeterol (as salmeterol xinafoate) to two healthy individuals gave peak plasma salmeterol concentrations of about 650 μg/L at about 45 minutes. In another volunteer, t1 ?2β was about 5.5 hours. The dose-normalised Cmax values in comparative animal studies suggest that the systemic absorption and bioavailability in humans is greater (about 15 times greater) than that in rodents, being more closely related to that in dogs. Absorption may be high in humans, and first-pass metabolism less than in animals[1].
Clinical Use
Salmeterol usually is prescribed for severe persistent asthma following previous treatment with a short-acting β-agonist, such as albuterol. The noticeable differences between salmeterol and albuterol are the onset of action and their duration of action (Table 13.6). When used regularly every day as prescribed, inhaled albuterol decreases the number and severity of asthma attacks. It is not used, however, for relieving an asthma attack that has already started.
Side Effects
Hoarseness, throat irritation, headache, rapid heartbeat, nervousness, cough, dry mouth/throat, or upset stomach may occur. To relieve dry mouth, suck on (sugarless) hard candy or ice chips, chew (sugarless) gum, drink water, or use a saliva substitute. This medication may raise your blood pressure. Rarely, this medication may cause sudden breathing problems/asthma right after you use it. If this occurs, use your quick-relief inhaler and get medical help right away.
Drug interactions
Many drugs besides salmeterol may affect the heart rhythm (QT prolongation), including amiodarone, dofetilide, pimozide, procainamide, quinidine, sotalol, macrolide antibiotics (such as erythromycin), among others. Before using salmeterol, report all medications you are currently using to your doctor or pharmacist.Other medications can affect the removal of salmeterol from your body, which may affect how salmeterol works. Examples include cobicistat, nefazodone, ritonavir, telithromycin, azole antifungals (such as ketoconazole, itraconazole), macrolide antibiotics (such as clarithromycin), HIV protease inhibitors (such as saquinavir), among others.
Metabolism
Salmeterol is predominantly metabolized by CYP3A4 to alpha-hydroxysalmeterol, and minorly by an unknown mechanism to an O-dealkylated metabolite.
Metabolism
Salmeterol is metabolized predominantly through CYP3A4, an isoform of cytochrome P450. CYP3A4 is responsible for the aliphatic oxidation of the salmeterol base. Salmeterol is extensively metabolized by hydroxylation into alpha-hydroxy-salmeterol and subsequently eliminated through feces and urine. Salmeterol is 57.4% eliminated in the feces and 23% in the urine. At recommended doses, systemic concentrations of salmeterol are low or undetectable. Only at very high doses are blood concentrations increased.
References
[1] M. Cazzola, M. Matera, R. Testi. “Clinical Pharmacokinetics of Salmeterol.” Clinical Pharmacokinetics 41 1 (2002): 19–30.
Properties of Salmeterol
| Melting point: | 75.5-76.5° |
| Boiling point: | 603.0±55.0 °C(Predicted) |
| Density | 1.112±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 9.99±0.31(Predicted) |
| form | Solid |
| color | White to Off-White |
| CAS DataBase Reference | 89365-50-4(CAS DataBase Reference) |
Safety information for Salmeterol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Salmeterol
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 89365-50-4 Salmeterol 98%View Details
89365-50-4 Salmeterol 98%View Details
89365-50-4 -
 Salmeterol 98.00% CAS 89365-50-4View Details
Salmeterol 98.00% CAS 89365-50-4View Details
89365-50-4 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
