EUK 134
Synonym(s):(SP-5-13)-Chloro[[2,2′-[1,2-ethanediylbis[(nitrilo-?N)methylidyne]]bis[6-methoxyphenolato-K O]](2-)]-manganese;2,2′-[1,2-Ethanediylbis(nitrilomethylidyne)]bis[6-methoxy-phenol manganese complex;Chloro[[2,2′-[1,2-ethanediylbis(nitrilomethylidyne)]bis[6-methoxyphenolato]](2-)-N2,N2′,O1,O1′]-manganese;EUK 134
- CAS NO.:81065-76-1
- Empirical Formula: C18H18ClMnN2O4
- Molecular Weight: 416.74
- MDL number: MFCD08059577
- EINECS: 000-000-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-14 20:10:21
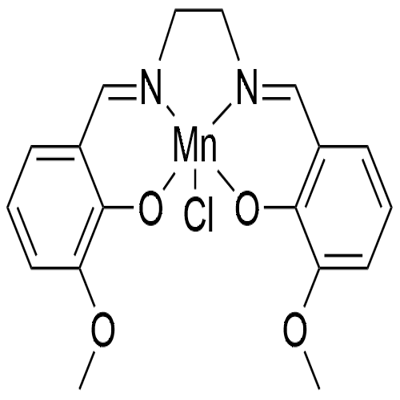
What is EUK 134 ?
The Uses of EUK 134
EUK-134 has been used as an antioxidant.
What are the applications of Application
EUK 134 is a salen-manganese complex, superoxide dismutase mimetic
Benefits
EUK-134 has been used as an antioxidant. An antioxidant serum that protects the skin from pollution and other external aggressors. In addition, it helps to reduce the appearance of redness and UV damage such as pigmentation and dark spots.
Biological Activity
euk 134, a synthetic superoxide dismutase (sod)/catalase mimetic, has exhibited potent antioxidant activities and inhibited the formation of β-amyloid and related amyloid fibril.
Biochem/physiol Actions
EUK-134 is a manganese-salen derivative that exhibit potent antioxidant activities. EUK-134 is a superoxide dismutase (SOD) that inhibits amyloid fibril formation, including islet amyloid polypeptide (IAPP), β-amyloid and lysozyme amyloid aggregation. It appears that EUK-134 disrupts the pre-formed amyloid fibrils. EUK-134 increases the viability of the SK-N-MC cells exposed to preformed IAPP fibrils.
in vitro
euk-134, a salen-manganese complex, showed potent catalase and cytoprotective activities and sod activity. after middle cerebral artery occlusion, euk-134 administration at 3 hr significantly reduced brain infarct size, with the highest dose apparently preventing further infarct growth[1]. administration of euk 134 (20 μm) prevented aβ-induced microglial proliferation in vitro[2]. in human neuroblastoma cell line sk-n-mc, pre-treatments with euk134 protected cells against h2o2-induced oxidative stress through inhibition of mapk pathway in a dose-dependent manner.euk134 also decreased the expression of pro-apoptotic genes p53 and bax and enhanced expression of anti-apoptotic bcl-2 gene [3]. incubation of human amylin with euk-134 significantly inhibited amyloid formation at two molar ratios of 1:1 and 5:1 (drugs to protein)[4].
in vivo
compared to the vehicle-injected rats, the euk-134-treated group at doses of 0.5 and 5.0 μmol/kg (0.25 and 2.5 mg/kg, respectively) exhibited infarct volumes that were significantly lower than those of vehicle-injected rats. at 5.0 μmol/kg, euk-134 reduced the infarct volume by 90% when compared with that of the vehicle controls [1].euk-134 protected most of the vulnerable neurons from excitotoxic cell death.euk-134 significantly reduced (p< 0.05) ka-induced neuronal damage in ca1 (22% of total neurons), an almost complete protection in ca3 (7%) and piriform cortex (14%), indicating that euk-134 prevented most but not all neuronal damage resulting from ka-induced seizure activity [5].
References
[1]. baker k, marcus c b, huffman k, et al. synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury[j]. journal of pharmacology and experimental therapeutics, 1998, 284(1): 215-221.
[2]. jekabsone a, mander p k, tickler a, et al. fibrillar beta-amyloid peptide aβ 1–40 activates microglial proliferation via stimulating tnf-α release and h 2 o 2 derived from nadph oxidase: a cell culture study[j]. journal of neuroinflammation, 2006, 3(1): 1.
[3]. mohammadi m, yazdanparast r. modulation of h2o2‐induced mitogen‐activated protein kinases activation and cell death in sk‐n‐mc cells by euk134, a salenderivative[j]. basic & clinical pharmacology & toxicology, 2011, 108(6): 378-384.
[4]. bahramikia s, yazdanparast r. inhibition of human islet amyloid polypeptide or amylin aggregation by two manganese-salenderivatives[j]. european journal of pharmacology, 2013, 707(1): 17-25.
[5]. rong y, doctrow s r, tocco g, et al. euk-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology[j]. proceedings of the national academy of sciences, 1999, 96(17): 9897-9902.
Properties of EUK 134
| storage temp. | -20°C |
| solubility | H2O: soluble2mg/mL, clear (warmed) |
| form | powder |
| color | , faint to very dark brown |
| InChI | InChI=1S/C18H20N2O4.ClH.Mn/c1-23-15-7-3-5-13(17(15)21)11-19-9-10-20-12-14-6-4-8-16(24-2)18(14)22;;/h3-8,11-12,21-22H,9-10H2,1-2H3;1H;/q;;+3/p-3/b19-11+,20-12+;; |
Safety information for EUK 134
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for EUK 134
| InChIKey | YUZJJFWCXJDFOQ-GAMUHHASSA-K |
| SMILES | [Cl-][Mn+3]123[O-]C4C(=CC=CC=4C=N1CCN2=CC1=CC=CC(OC)=C1[O-]3)OC |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Salen-Mn 98.00% CAS 81065-76-1View Details
Salen-Mn 98.00% CAS 81065-76-1View Details
81065-76-1 -
 EUK-134 CAS 81065-76-1View Details
EUK-134 CAS 81065-76-1View Details
81065-76-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1