S-Acetyl-L-glutathione
- CAS NO.:3054-47-5
- Empirical Formula: C12H19N3O7S
- Molecular Weight: 349.36016
- MDL number: MFCD09952588
- EINECS: 2212755
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-14 17:34:16
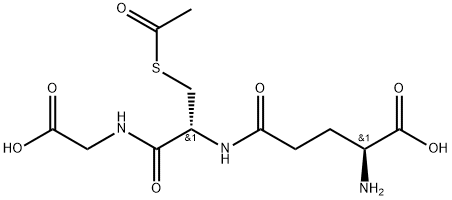
What is S-Acetyl-L-glutathione?
Description
S-acetyl-L-glutathione is a glutathione derivative that can increase intracellular glutathione similar to a prodrug. It can be used to replenish the depletion of reduced glutathione occurring during HIV infection and to inhibit HIV replication. However, it was found to selectively induce apoptosis in human lymphoma cells, Daudi, Raji and Jurkat cells, by depleting the intracellular glutathione level rather than increase it.
The Uses of S-Acetyl-L-glutathione
S-Acetyl L-Glutathione is used for treating cataracts, glaucoma, preventing aging, treating or preventing alcoholism, asthma, cancer, heart disease (atherosclerosis and hypercholesterolemia), hepatitis, liver disease, immunosuppression (including AIDS and Chronic Fatigue Syndrome), maintaining immune function, memory loss, Alzheimer’s disease, osteoarthritis, Parkinson’s disease, people with ASD, detoxifying metals and drugs, cystic fibrosis, for preventing toxicity of chemotherapy, for treating male infertility, for preventing anaemia in patients undergoing haemodialysis, preventing renal dysfunction after coronary bypass surgery, shown to selectively induce apoptosis (cell death) in human lymphoma cancer cells.
The Uses of S-Acetyl-L-glutathione
Research has found that addition of S-acetyl glutathione to the medium raised intracellular glutathione content material in cultured fibroblasts from patients with glutathione synthetase deficiency. This found is important to the treatment of patients with this inborn blunders of metabolism. The S-Acetyl Glutathione supplement is one of the best medications to help balance the overall functions of the human body. Especially for athletes, non-athletes, and bodybuilders out there.
Definition
The key difference between l glutathione and S-Acetyl-L-glutathione is that l-glutathione is the abundant isomer form of glutathione, whereas S-Acetyl-L-glutathione is a derivative of glutathione. Unlike glutathione, S-Acetyl-L-glutathione is absorbed well orally.
Benefits
S-Acetyl glutathione has been found to increase intracellular glutathione and improve many biomarkers of oxidative stress. Taking S-Acetyl Glutathione supplement is a great way to protect ourselves from health problems associated with aging, poor lifestyle and adverse environmental factors. Also, it has additional benefits as follows:
- Provides Antioxidant Support
- Healthy Cell Function and Healthy Aging
- Detoxification
- Healthy Immune Response
- Transport of Amino Acids to Cells
- Enhances Antioxidant Activity of Vitamins C and E
- Increases Energy Production
- Overall Health and Energy
Biological Functions
S-Acetyl L-Glutathione is the most effective glutathione variant currently on the market. Glutathione (Gluthathione) is one of the most potent antioxidants that is naturally produced by the body (and the only one that is intracellular) and it has been shown to neutralize free radicals, detoxify the liver, and to improve the functioning of the immune system. After age 45, the body begins to produce less and less Glutathione and supplementing it becomes an attractive idea. Unfortunately, most Glutathione supplements have nearly no bioavailability, as the molecule breaks down rapidly after oral ingestion. S-Acetyl L-Glutathione is an altered form with an attached acetyl function group. This greatly improves its ability to remain intact in the gut and allows a greater concentration to be absorbed into the bloodstream where it can take effect. Double Wood Supplement’s S-Acetyl Glutathione is manufactured in New York city and tested for purity right here in the USA.
Biological Activity
S-Acetyl-L-glutathione is a derivative of glutathione (GSH). It is more stable in plasma than GSH and, unlike GSH, can be taken up into cells, where it is converted to GSH by intracellular thioesterases. S-Acetyl-L-glutathione (50 μM) increases intracellular GSH levels in primary fibroblasts derived from patients with glutathione synthetase deficiency.1 It induces apoptosis in Daudi, Raji, and Jurkat lymphoma cells when used at a concentration of 5 mM. It inhibits the replication of herpes simplex virus 1 (HSV-1) in human foreskin fibroblasts when used at concentrations of 5 and 10 mM. S-Acetyl-L-glutathione (6.25 μg/g per day), but not GSH, increases survival in a mouse model of HSV-1 infection.
Side Effects
S-Acetyl L-Glutathione is very well tolerated when taken at the recommended dosage and adverse side effects are very rarely reported. Long term supplementation of glutathione has been shown to lower zinc levels.
While some studies have shown that inhaled glutathione can trigger asthma attacks in people suffering from asthma, there is not currently enough evidence to show that this applies to orally ingested glutathione. Asthma sufferers may want to play it safe and avoid using Glutathione supplements. Please consult with your physician before taking S-Acetyl L-Glutathione.
Overdosage
The recommended dose is 500 mg of S-Acetyl L-Glutathione per day. We would suggest you integrate it with other supplements to truly maximize the absorption, lifespan, recycling, and benefits of S-Acetyl L-Glutathione. A few big name helpers are Coenzyme Q10 (CoQ10), Selenium, Vitamin C, A and E, as well B-complex, B12 (methylcobalamin) and Trans-Resveratrol.
Mode of action
S-Acetylglutathione (SAG) is a GSH precursor, it is more stable than GSH itself in plasma and is taken up directly by cells and later converted to GSH (see Figure 1). The acetylation of the sulfur atom prevents the decomposition of GSH and facilitates its absorption through the intestinal wall as is, thus enabling the molecule to pass extensively into the cells. Moreover, cysteinyl acetylation prevents the oxidation of the thiol group before its absorption. After absorption, SAG is hydrolyzed by cytoplasmic thioesterases, so releasing a GSH pool available for the cells. The addition of SAG to cultures of fibroblasts originating from individuals suffering from a genetic glutathione synthetase deficiency has proved able to replenish the intracellular level of GSH effectively. SAG has proven to be more stable in plasma and more effective than GSH in replenishing the cell levels of GSH depleted by viral infections. Moreover, SAG exhibits an interesting non-GSH-dependent activity that induces apoptosis in some human tumor cell lines in vitro.
Figure 1: Biochemical pathway of SAG and GSH.
References
Effects of S-acetylglutathione in cell and animal model of herpes simplex virus type 1 infection
S-Acetylglutathione normalizes intracellular glutathione content in cultured fibroblasts from patients with glutathione synthetase deficiency
S-acetyl-glutathione selectively induces apoptosis in human lymphoma cells through a GSH-independent mechanism
Properties of S-Acetyl-L-glutathione
| Boiling point: | 770.2±60.0 °C(Predicted) |
| Density | 1.436±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | PBS (pH 7.2): 1 mg/ml |
| pka | 2.21±0.10(Predicted) |
| form | A crystalline solid |
| InChI | InChI=1/C12H19N3O7S/c1-6(16)23-5-8(11(20)14-4-10(18)19)15-9(17)3-2-7(13)12(21)22/h7-8H,2-5,13H2,1H3,(H,14,20)(H,15,17)(H,18,19)(H,21,22)/t7-,8-/s3 |
Safety information for S-Acetyl-L-glutathione
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for S-Acetyl-L-glutathione
| InChIKey | FVRWSIPJNWXCEO-KWTCHIRYNA-N |
| SMILES | [C@H](CSC(=O)C)(C(=O)NCC(=O)O)NC(=O)CC[C@H](N)C(=O)O |&1:0,18,r| |
S-Acetyl-L-glutathione manufacturer
Siddhi Vinayaka Spechem Private Limited
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
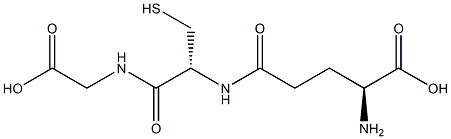


You may like
-
 S-Acetylglutathione 3054-47-5 / 4754-38-5 99%View Details
S-Acetylglutathione 3054-47-5 / 4754-38-5 99%View Details
3054-47-5 / 4754-38-5 -
 3054-47-5 / 4754-38-5 99%View Details
3054-47-5 / 4754-38-5 99%View Details
3054-47-5 / 4754-38-5 -
 Sag 95% CAS 3054-47-5View Details
Sag 95% CAS 3054-47-5View Details
3054-47-5 -
 Powder S-Acetyl-L-Glutathione, For Fairness cream manufacturing, 25 kg BagView Details
Powder S-Acetyl-L-Glutathione, For Fairness cream manufacturing, 25 kg BagView Details
3054-47-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
