Ruthenium carbonyl
Synonym(s):Ruthenium carbonyl;tri-Ruthenium dodecacarbonyl
- CAS NO.:15243-33-1
- Empirical Formula: C12O12Ru3
- Molecular Weight: 639.33
- MDL number: MFCD00011209
- EINECS: 239-287-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:07:28
What is Ruthenium carbonyl?
Description
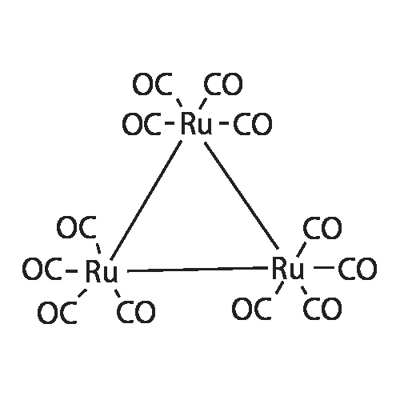
Triruthenium dodecacarbonyl [Ru3(CO)12] is a coordination compound that consists of a triangle of ruthenium atoms, each of which is bonded to four carbon monoxide molecules. It appears as dark orange crystals that are soluble in nonpolar solvents but not in water.
Ru3(CO)12 and the similar osmium dodecacarbonyl1 [Os3(CO)12] were originally reported to have the formulas Ru2(CO)9 and Os2(CO)9, respectively. But an extensive analysis in 1961 by Eugene E. Corey and Lawrence F. Dahl* at the University of Wisconsin (Madison) revealed the previously unforeseen trigonal structures of the molecules. The authors used crystallographic and spectroscopic evidence to establish the molecular structures.
Ru3(CO)12 is synthesized via the base-catalyzed liquid-phase reaction of ruthenium trichloride2 (RuCl3) with CO. It also has been made under high CO pressure from ruthenium stearate3 dissolved in cyclohexane or from RuCl3 and metallic zinc in ethanol.
The primary use for Ru3(CO)12 is the synthesis of other ruthenium complexes, many with CO combined with other ligands such as alkenes. Two recent patents to Tokyo Electron Ltd. (Tokyo) describe its use for making ruthenium films deposited on silicon substrates that form semiconductors.
This year, Ru3(CO)12 has also been used for an educational purpose. It, along with six other inorganic molecules with varying types of symmetry, were examples used by Donald J. Wink and collaborators at the University of Illinois Chicago to instruct students to recognize symmetry elements. The activity included student-created 2-D drawings and 3-D molecular models.
Considering that CO and most ruthenium compounds are highly toxic, Ru3(CO)12 exhibits remarkably few hazards.
1. CAS Reg. No. 15696-40-9.
2. CAS Reg. No. 10049-08-8.
3. CAS Reg. No. 153728-81-5.
Description
Triruthenium dodecacarbonyl is a metal carbonyl complex, classified as an inorganic compound. Its molecular formula is Ru3(CO)12, with a molecular weight of 639.33. It possesses D3h symmetry and a core composed of a Ru equilateral triangle. Each Ru atom is attached to two axial and two equatorial CO ligands. Unlike triiron dodecacarbonyl, this compound does not contain any bridge bonds. It is commonly employed as a precursor for various organoruthenium compounds.
Chemical properties
The substance appears as orange plates or powder and is soluble in non-polar organic solvents.
The Uses of Ruthenium carbonyl
A H-transfer catalyst. It is used in carbonylation catalysis of the allylic amination of unactivated olefins by nitroarenes, reductive carbonylation of mononitro aromatic compounds to carbamates, and as a catalyst for cycloaddition reactions. It is employed as a precursor for the synthesis of Ru nanoparticles. Catalyst used in the intermolecular [3+2+1] cycloaddition of alpha,beta-unsaturated ketones with silylacetylenes and carbon monoxide, to produce alpha-pyrones. Catalyzes the coupling of silanes with thiols, alcohols and amines with high turnover number (TON) and high turnover frequency (TOF).
What are the applications of Application
Triruthenium dodecacarbonyl is an H-transfer catalyst and carbonyl cluster precursor
Reactions
- Catalyst for the conversion of enynes to catechol derivatives.
- Catalyst for the intermolecular [2+2+1] cycloaddition of ketones, CO and alkenes or alkynes.
- 3-Component couplings.
- Reaction of α,β-unsaturated imines with carbon monoxide and alkenes to form β,γ-unsaturated γ-butyroactams.
- Ester decarboxylation.
- Catalyst for hydroamination and C-H bond activation.
- Used in sp3 C-H bond arylation and carbonylation.
- Ru/halide catalytic system for C-C bond forming reaction between alkynes and unsaturated carbonyl compounds.
- Amination of α-hydroxy amides.


General Description
Atomic number of base material: 44 Ruthenium
Properties of Ruthenium carbonyl
| Melting point: | 150 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Sparingly soluble in hydrocarbons (e.g. hexane, cyclohexane, benzene) and acetone. |
| form | crystal |
| appearance | orange crystals or powder |
| color | orange |
| CAS DataBase Reference | 15243-33-1(CAS DataBase Reference) |
| EPA Substance Registry System | Ruthenium, dodecacarbonyltri-, triangulo (15243-33-1) |
Safety information for Ruthenium carbonyl
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. |
Computed Descriptors for Ruthenium carbonyl
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 15243-33-1 DIRUTHENIUM DODECACARBONYL 99%View Details
15243-33-1 DIRUTHENIUM DODECACARBONYL 99%View Details
15243-33-1 -
 Triruthenium Dodecacarbonyl CAS 15243-33-1View Details
Triruthenium Dodecacarbonyl CAS 15243-33-1View Details
15243-33-1 -
 Triruthenium dodecacarbonyl CAS 15243-33-1View Details
Triruthenium dodecacarbonyl CAS 15243-33-1View Details
15243-33-1 -
 Triruthenium dodecacarbonyl powderView Details
Triruthenium dodecacarbonyl powderView Details
15243-33-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
