(R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE
Synonym(s):(R,R)-Jacobsen’s catalyst;Jacobsen’s catalyst
- CAS NO.:138124-32-0
- Empirical Formula: C36H52ClMnN2O2
- Molecular Weight: 635.2
- MDL number: MFCD02101664
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-13 18:16:27
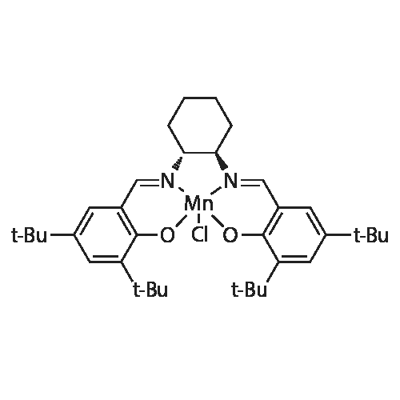
What is (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE?
Description
Jacobsen’s catalyst is an Mn(III)-salen Schiff base complex. It is used as a catalyst in asymmetric syntheses, including the epoxidation of olefins.
Chemical properties
(R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE is Dark brown powder or chunks
The Uses of (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE
(R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE is a coordinated compound of manganese and a salen-type ligand. It is also used as an asymmetric catalyst used to enantioselectively transform prochiral alkenes into epoxides.
The Uses of (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE
Chiral catalyst for epoxidation of olefins.
The Uses of (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE
Salen (Mn)-catalyzed asymmetric epoxidation of unfunctionalized olefins. High enantioselectivities and yields are obtained for a variety of substrates, especially cis-alkenes. A few applications include the synthesis of the taxol side chain and cis-1-amino-2-indanol. Jacobsen′s catalyst has also been used in the asymmetric α-hydroxylation of silyl enol ethers. Catalyst for the enantioselective epoxidation of a variety of olefins.
What are the applications of Application
(R,R)-Jacobsen′s catalyst is a chiral compound used as a catalytic reagent
Reactions
- Catalyst for the conversion of olefins to chiral epoxides in high enantiomeric excess.
- Pharmaceutically important, single enantiomer amino alcohols are efficiently produced from the corresponding chiral epoxide by acid or base-catalyzed epoxide ring-opening reactions.
- symmetric Kinetic resolution of secondary alcohols in water.
- Enantioselective Reformatsky reaction with ketones.

General Description
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information.
Properties of (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE
| Melting point: | 330-332 °C(lit.) |
| alpha | D23 +580° (c = 0.01 in ethanol) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| form | Liquid May Develop Some Turbidity or Precipitate |
| color | Light yellow to gold to brown |
| λmax | 509nm(CH2Cl2)(lit.) |
| Merck | 14,5252 |
Safety information for (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINO-MANGANESE(III) CHLORIDE
| InChIKey | LJVAWOSDJSQANR-OHRASPNLSA-K |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![(1R,2R)-(-)-[1,2-CYCLOHEXANEDIAMINO-N N'-BIS(3,5-DI-T-BUTYLSALICYLIDENE)]CHROMIUM (III) CHLORIDE](https://img.chemicalbook.in/CAS/GIF/164931-83-3.gif)

You may like
-
 138124-32-0 (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINOMANGANESE(III)CHLORIDE 99%View Details
138124-32-0 (R,R)-(-)-N,N'-BIS(3,5-DI-TERT-BUTYLSALICYLIDENE)-1,2-CYCLOHEXANEDIAMINOMANGANESE(III)CHLORIDE 99%View Details
138124-32-0 -
 (R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) Chloride CAS 138124-32-0View Details
(R,R)-(-)-N,N'-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) Chloride CAS 138124-32-0View Details
138124-32-0 -
 (R,R)-(−)-N,N′-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride CAS 138124-32-0View Details
(R,R)-(−)-N,N′-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride CAS 138124-32-0View Details
138124-32-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8