Rosuvastatin IMpurity SodiuM Salt (5-Oxo Rosuvastatin SodiuM Salt)
- CAS NO.:1422619-13-3
- Empirical Formula: C22H26FN3O6S
- Molecular Weight: 479.52
- MDL number: MFCD18382415
- EINECS: 604-604-1
- Update Date: 2023-09-06 17:30:42
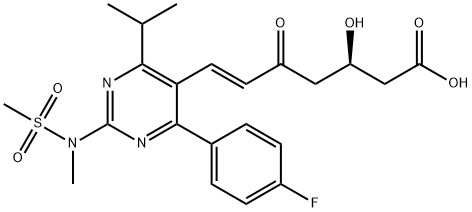
What is Rosuvastatin IMpurity SodiuM Salt (5-Oxo Rosuvastatin SodiuM Salt)?
The Uses of Rosuvastatin IMpurity SodiuM Salt (5-Oxo Rosuvastatin SodiuM Salt)
5-Oxo Rosuvastatin (Rosuvastatin EP Impurity C (Calcium Salt)) is a Rosuvastatin (R700500) impurity.
What are the applications of Application
5-Oxo Rosuvastatin (Rosuvastatin EP Impurity C (Calcium Salt)) is a Rosuvastatin impurity. It can be used as a reference substance to analyze the quality of drugs and is often used in the pharmaceutical industry. Rosuvastatin is used together with a proper diet to lower bad cholesterol (LDL) and triglycerides (fats) in the blood and to increase good cholesterol (HDL). It is also used to treat adults who cannot control their cholesterol levels by diet and exercise alone. It may help prevent or slow down medical problems, like atherosclerosis (hardening of the arteries), that are caused by fats clogging the blood vessels. It may also be used to prevent certain types of heart and blood vessel problems in patients with risk factors for heart problems.
Properties of Rosuvastatin IMpurity SodiuM Salt (5-Oxo Rosuvastatin SodiuM Salt)
| Boiling point: | 731.0±70.0 °C(Predicted) |
| Density | 1.365±0.06 g/cm3(Predicted) |
| solubility | Chloroform (Slightly), DMSO (Sparingly), Methanol (Sparingly) |
| pka | 4.17±0.10(Predicted) |
| form | Solid |
| color | Pale Yellow to Very Dark Beige |
| Stability: | Hygroscopic |
Safety information for Rosuvastatin IMpurity SodiuM Salt (5-Oxo Rosuvastatin SodiuM Salt)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Rosuvastatin IMpurity SodiuM Salt (5-Oxo Rosuvastatin SodiuM Salt)
| InChIKey | SGVASDMKSZUTTN-OAGJVSPASA-N |
| SMILES | C(O)(=O)C[C@H](O)CC(=O)/C=C/C1=C(C(C)C)N=C(N(C)S(C)(=O)=O)N=C1C1=CC=C(F)C=C1 |
Rosuvastatin IMpurity SodiuM Salt (5-Oxo Rosuvastatin SodiuM Salt) manufacturer
Varanous Labs Pvt Ltd
Vishrudh laboratories pvt ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
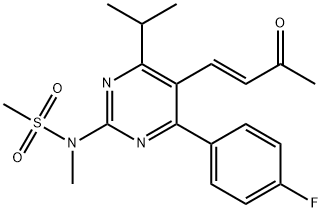
 1422619-13-3 Rosuvastatin EP Impurity C; 5-Oxo Rosuvastatin; Rosuvastatin B2 98%View Details
1422619-13-3 Rosuvastatin EP Impurity C; 5-Oxo Rosuvastatin; Rosuvastatin B2 98%View Details
1422619-13-3 -
 Rosuvastatin 5-Keto acid impurity 98%View Details
Rosuvastatin 5-Keto acid impurity 98%View Details
1422619-13-3 -
 Rosuvastatin 5-keto acid impurity 1422619-13-3. 98%View Details
Rosuvastatin 5-keto acid impurity 1422619-13-3. 98%View Details
1422619-13-3. -
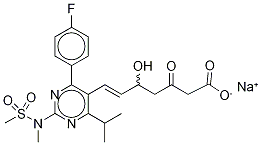
 1422619-13-3 5-Oxo Rosuvastatin 98%View Details
1422619-13-3 5-Oxo Rosuvastatin 98%View Details
1422619-13-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1