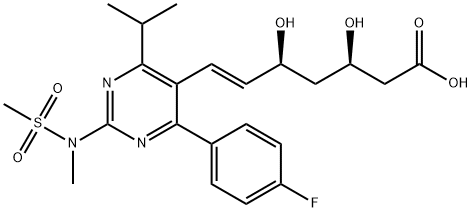
Rosuvastatin
- CAS NO.:287714-41-4
- Empirical Formula: C22H28FN3O6S
- Molecular Weight: 481.54
- MDL number: MFCD08460959
- EINECS: 689-191-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
What is Rosuvastatin?
Absorption
In a study of healthy white male volunteers, the absolute oral bioavailability of rosuvastatin was found to be approximately 20% while absorption was estimated to be 50%, which is consistent with a substantial first-pass effect after oral dosing. Another study in healthy volunteers found that the peak plasma concentration (Cmax) of rosuvastatin was 6.06ng/mL and was reached at a median of 5 hours following oral dosing. Both Cmax and AUC increased in approximate proportion to dose. Neither food nor evening versus morning administration was shown to have an effect on the AUC of rosuvastatin. Many statins are known to interact with hepatic uptake transporters and thus reach high concentrations at their site of action in the liver.
Breast Cancer Resistance Protein (BCRP) is a membrane-bound protein that plays an important role in the absorption of rosuvastatin, particularly as CYP3A4 has minimal involvement in its metabolism. Evidence from pharmacogenetic studies of c.421C>A single nucleotide polymorphisms (SNPs) in the gene for BCRP has demonstrated that individuals with the 421AA genotype have reduced functional activity and 2.4-fold higher AUC and Cmax values for rosuvastatin compared to study individuals with the control 421CC genotype. This has important implications for the variation in response to the drug in terms of efficacy and toxicity, particularly as the BCRP c.421C>A polymorphism occurs more frequently in Asian populations than in Caucasians. Other statin drugs impacted by this polymorphism include fluvastatin and atorvastatin.
Genetic differences in the OATP1B1 (organic-anion-transporting polypeptide 1B1) hepatic transporter have also been shown to impact rosuvastatin pharmacokinetics. Evidence from pharmacogenetic studies of the c.521T>C SNP showed that rosuvastatin AUC was increased 1.62-fold for individuals homozygous for 521CC compared to homozygous 521TT individuals. Other statin drugs impacted by this polymorphism include simvastatin, pitavastatin, atorvastatin, and pravastatin.
For patients known to have the above-mentioned c.421AA BCRP or c.521CC OATP1B1 genotypes, a maximum daily dose of 20mg of rosuvastatin is recommended to avoid adverse effects from the increased exposure to the drug, such as muscle pain and risk of rhabdomyolysis.
Toxicity
Generally well-tolerated. Side effects may include myalgia, constipation, asthenia, abdominal pain, and nausea. Other possible side effects include myotoxicity (myopathy, myositis, rhabdomyolysis) and hepatotoxicity. To avoid toxicity in Asian patients, lower doses should be considered. Pharmacokinetic studies show an approximately two-fold increase in peak plasma concentration and AUC in Asian patients (Philippino, Chinese, Japanese, Korean, Vietnamese, or Asian-Indian descent) compared to Caucasian patients.
Description
Rosuvastatin is the most recent FDA-approved statin (doses 5–40 mg) for the treatment of dyslipidemia. Rosuvastatin, which has a structure similar to other synthetic statins, has a long halflife (20–24 hours) similar to atorvastatin and hydrophilicity comparable to pravastatin. Consequently, rosuvastatin is minimally metabolized and has no significant cytochrome P450 drug interactions. The efficacy of rosuvastatin is superior to that of other statins. Compared to atorvastatin, rosuvastatin provides approximately an 8% additional lowering of LDL-C on an equivalent dose level. This provides modestly more efficacy than a doubling of the dose of atorvastatin. Rosuvastatin also increases HDL-C slightly more than atorvastatin, especially at the highest doses.
The Uses of Rosuvastatin
Rosuvastatin Calcium is a competitive inhibitor of HMG-CoA reductase with IC50 of 11 nM.
Background
Rosuvastatin, also known as the brand name product Crestor, is a lipid-lowering drug that belongs to the statin class of medications, which are used to lower the risk of cardiovascular disease and manage elevated lipid levels by inhibiting the endogenous production of cholesterol in the liver. More specifically, statin medications competitively inhibit the enzyme hydroxymethylglutaryl-coenzyme A (HMG-CoA) Reductase, which catalyzes the conversion of HMG-CoA to mevalonic acid and is the third step in a sequence of metabolic reactions involved in the production of several compounds involved in lipid metabolism and transport including cholesterol, low-density lipoprotein (LDL) (sometimes referred to as "bad cholesterol"), and very low-density lipoprotein (VLDL). Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD, such as those with Type 2 Diabetes. The clear evidence of the benefit of statin use coupled with very minimal side effects or long term effects has resulted in this class becoming one of the most widely prescribed medications in North America.
Rosuvastatin and other drugs from the statin class of medications including atorvastatin, pravastatin, simvastatin, fluvastatin, and lovastatin are considered first-line options for the treatment of dyslipidemia. This is largely due to the fact that cardiovascular disease (CVD), which includes heart attack, atherosclerosis, angina, peripheral artery disease, and stroke, has become a leading cause of death in high-income countries and a major cause of morbidity around the world. Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks.
While all statin medications are considered equally effective from a clinical standpoint, rosuvastatin is considered the most potent; doses of 10 to 40mg rosuvastatin per day were found in clinical studies to result in a 45.8% to 54.6% decreases in LDL cholesterol levels, which is about three-fold more potent than atorvastatin's effects on LDL cholesterol. However, the results of the SATURN trial concluded that despite this difference in potency, there was no difference in their effect on the progression of coronary atherosclerosis.
Rosuvastatin is also a unique member of the class of statins due to its high hydrophilicity which increases hepatic uptake at the site of action, low bioavailability, and minimal metabolism via the Cytochrome P450 system. This last point results in less risk of drug-drug interactions compared to atorvastatin, lovastatin, and simvastatin, which are all extensively metabolized by Cytochrome P450 (CYP) 3A4, an enzyme involved in the metabolism of many commonly used drugs. Drugs such as ciclosporin, gemfibrozil, and some antiretrovirals are more likely to interact with this statin through antagonism of OATP1B1 organic anion transporter protein 1B1-mediated hepatic uptake of rosuvastatin.
Indications
The FDA monograph states that rosuvastatin is indicated as an adjunct to diet in the treatment of triglyceridemia, Primary Dysbetalipoproteinemia (Type III Hyperlipoproteinemia), and Homozygous Familial Hypercholesterolemia.
The Health Canada monograph for rosuvastatin further specifies that rosuvastatin is indicated for the reduction of elevated total cholesterol (Total-C), LDL-C, ApoB, the Total-C/HDL-C ratio and triglycerides (TG) and for increasing HDL-C in hyperlipidemic and dyslipidemic conditions when response to diet and exercise alone has been inadequate. It is also indicated for the prevention of major cardiovascular events (including risk of myocardial infarction, nonfatal stroke, and coronary artery revascularization) in adult patients without documented history of cardiovascular or cerebrovascular events, but with at least two conventional risk factors for cardiovascular disease.
Prescribing of statin medications is considered standard practice following any cardiovascular events and for people with a moderate to high risk of development of CVD. Statin-indicated conditions include diabetes mellitus, clinical atherosclerosis (including myocardial infarction, acute coronary syndromes, stable angina, documented coronary artery disease, stroke, trans ischemic attack (TIA), documented carotid disease, peripheral artery disease, and claudication), abdominal aortic aneurysm, chronic kidney disease, and severely elevated LDL-C levels.
Definition
ChEBI: Rosuvastatin is a dihydroxy monocarboxylic acid that is (6E)-7-{4-(4-fluorophenyl)-2-[methyl(methylsulfonyl)amino]-6-(propan-2-yl)pyrimidin-5-yl} hept-6-enoic acid carrying two hydroxy substituents at positions 3 and 5 (the 3R,5S-diastereomer). It has a role as an antilipemic drug, an anti-inflammatory agent, a CETP inhibitor, a cardioprotective agent, a xenobiotic and an environmental contaminant. It is a member of pyrimidines, a sulfonamide, a dihydroxy monocarboxylic acid, a statin (synthetic) and a member of monofluorobenzenes. It derives from a hept-6-enoic acid. It is a conjugate acid of a rosuvastatin(1-).
Preparation
Rosuvastatin synthesis: Reduction to the 5-hydroxymethyl derivative proceeds smoothly with diisobutylaluminum hydride (DIBALH) in toluene at -10 °C. The hydroxymethyl group is then converted to the bromo derivative, which upon reaction with triphenylphosphine affords the Wittig reagent. The latter is treated with tert-butyl 2-[(4R,6S)-6-formyl-2,2-dimethyl-1,3-dioxan-4-yl]acetate and provides the protected rosuvastatin ester. Removal of the dioxane protecting group by HCl, ester hydrolysis with NaOH and precipitation with CaCl2 gives rosuvastatin.
Kleemann A; Cardiovascular Drugs. Ullmann's Encyclopedia of Industrial Chemistry 7th ed. (1999-2017). NY, NY: John Wiley & Sons. Online Posting Date: January 15, 2008
brand name
Crestor (AstraZeneca).
General Description
Rosuvastatin, 7-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-(methyl-methylsulfonyl-amino)-pyrimidin-5-yl]-3,5-dihydroxy-hept-6-enoic acid (Crestor), is oneof the more recently introduced statins in the United States.As with all statins, there is a concern of rhabdomyolysis andas such, the FDA has mandated that a warning about thisside effect, as well as a kidney toxicity warning, be added tothe product label (http://www.fda.gov/CDER/Drug/advisory/crestor_3_2005.htm). This should not come as a surprisebecause of the relationship in the chemical architectureto cerivastatin, which was withdrawn from the market as aresult of its adverse side effects.
Pharmacokinetics
Rosuvastatin is a synthetic, enantiomerically pure antilipemic agent. It is used to lower total cholesterol, low density lipoprotein-cholesterol (LDL-C), apolipoprotein B (apoB), non-high density lipoprotein-cholesterol (non-HDL-C), and trigleride (TG) plasma concentrations while increasing HDL-C concentrations. High LDL-C, low HDL-C and high TG concentrations in the plasma are associated with increased risk of atherosclerosis and cardiovascular disease. The total cholesterol to HDL-C ratio is a strong predictor of coronary artery disease and high ratios are associated with higher risk of disease. Increased levels of HDL-C are associated with lower cardiovascular risk. By decreasing LDL-C and TG and increasing HDL-C, rosuvastatin reduces the risk of cardiovascular morbidity and mortality.
Elevated cholesterol levels, and in particular, elevated low-density lipoprotein (LDL) levels, are an important risk factor for the development of CVD. Use of statins to target and reduce LDL levels has been shown in a number of landmark studies to significantly reduce the risk of development of CVD and all-cause mortality. Statins are considered a cost-effective treatment option for CVD due to their evidence of reducing all-cause mortality including fatal and non-fatal CVD as well as the need for surgical revascularization or angioplasty following a heart attack. Evidence has shown that even for low-risk individuals (with <10% risk of a major vascular event occurring within 5 years) statins cause a 20%-22% relative reduction in major cardiovascular events (heart attack, stroke, coronary revascularization, and coronary death) for every 1 mmol/L reduction in LDL without any significant side effects or risks.
Skeletal Muscle Effects
Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. These risks can occur at any dose level, but are increased at the highest dose (40 mg). Rosuvastatin should be prescribed with caution in patients with predisposing factors for myopathy (e.g., age ≥ 65 years, inadequately treated hypothyroidism, renal impairment).
The risk of myopathy during treatment with rosuvastatin may be increased with concurrent administration of some other lipid-lowering therapies (such as fenofibrate or niacin), gemfibrozil, cyclosporine, atazanavir/ritonavir, lopinavir/ritonavir, or simeprevir. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors, including rosuvastatin, coadministered with colchicine, and caution should therefore be exercised when prescribing these two medications together.
Real-world data from observational studies has suggested that 10-15% of people taking statins may experience muscle aches at some point during treatment.
Liver Enzyme Abnormalities
Increases in serum transaminases have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. In most cases, the elevations were transient and resolved or improved on continued therapy or after a brief interruption in therapy. There were two cases of jaundice, for which a relationship to rosuvastatin therapy could not be determined, which resolved after discontinuation of therapy. There were no cases of liver failure or irreversible liver disease in these trials.
Endocrine Effects
Increases in HbA1c and fasting serum glucose levels have been reported with HMG-CoA reductase inhibitors, including rosuvastatin calcium tablets. Based on clinical trial data with rosuvastatin, in some instances these increases may exceed the threshold for the diagnosis of diabetes mellitus.
An in vitro study found that atorvastatin, pravastatin, rosuvastatin, and pitavastatin exhibited a dose-dependent cytotoxic effect on human pancreas islet β cells, with reductions in cell viability of 32, 41, 34 and 29%, respectively, versus control]. Moreover, insulin secretion rates were decreased by 34, 30, 27 and 19%, respectively, relative to control.
HMG-CoA reductase inhibitors interfere with cholesterol synthesis and lower cholesterol levels and, as such, might theoretically blunt adrenal or gonadal steroid hormone production. Rosuvastatin demonstrated no effect upon nonstimulated cortisol levels and no effect on thyroid metabolism as assessed by TSH plasma concentration. In rosuvastatin treated patients, there was no impairment of adrenocortical reserve and no reduction in plasma cortisol concentrations. Clinical studies with other HMG-CoA reductase inhibitors have suggested that these agents do not reduce plasma testosterone concentration. The effects of HMG-CoA reductase inhibitors on male fertility have not been studied. The effects, if any, on the pituitarygonadal axis in premenopausal women are unknown.
Cardiovascular
Ubiquinone levels were not measured in rosuvastatin clinical trials, however significant decreases in circulating ubiquinone levels in patients treated with other statins have been observed. The clinical significance of a potential long-term statin-induced deficiency of ubiquinone has not been established. It has been reported that a decrease in myocardial ubiquinone levels could lead to impaired cardiac function in patients with borderline congestive heart failure.
Lipoprotein A
In some patients, the beneficial effect of lowered total cholesterol and LDL-C levels may be partly blunted by a concomitant increase in the Lipoprotein(a) [Lp(a)] concentrations. Present knowledge suggests the importance of high Lp(a) levels as an emerging risk factor for coronary heart disease. It is thus desirable to maintain and reinforce lifestyle changes in high-risk patients placed on rosuvastatin therapy. Further studies have demonstrated statins affect Lp(a) levels differently in patients with dyslipidemia depending on their apo(a) phenotype; statins increase Lp(a) levels exclusively in patients with the low molecular weight apo(a) phenotype.
Clinical Use
HMG CoA reductase inhibitor:
Hyperlipidaemia
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: concentration possibly increased
by dronedarone - reduce dose of rosuvastatin.
Antibacterials: erythromycin reduces concentration
of rosuvastatin; increased risk of myopathy with
daptomycin and fusidic acid - avoid.
Anticoagulants: effect of coumarins and phenindione
enhanced.
Antifungals: concentration increased by itraconazole
- reduce dose of rosuvastatin.
Antivirals: increased risk of myopathy with
atazanavir, darunavir, dasabuvir, fosamprenavir,
indinavir, ledipasvir, lopinavir, paritaprevir,
ritonavir, saquinavir and tipranavir - reduce dose
of rosuvastatin, avoid with fosamprenavir, indinavir,
ledipasvir, ritonavir and saquinavir.
Ciclosporin: increased risk of myopathy - avoid.
Clopidogrel: concentration of rosuvastatin increased,
maximum rosuvastatin dose is 20 mg in normal renal
function.
Colchicine: possible increased risk of myopathy.
Cytotoxics: concentration increased by eltrombopag
- reduce dose of rosuvastatin.
Lipid-lowering agents: increased risk of myopathy
with ezetimibe, fibrates, gemfibrozil (avoid) and
nicotinic acid - reduce dose of rosuvastatin.
Teriflunomide: concentration increased by
teriflunomide - reduce dose of rosuvastatin.
Metabolism
Rosuvastatin is not extensively metabolized, as demonstrated by the small amount of radiolabeled dose that is recovered as a metabolite (~10%). Cytochrome P450 (CYP) 2C9 is primarily responsible for the formation of rosuvastatin's major metabolite, N-desmethylrosuvastatin, which has approximately 20-50% of the pharmacological activity of its parent compound in vitro. However, this metabolic pathway isn't deemed to be clinically significant as there were no observable effects found on rosuvastatin pharmacokinetics when rosuvastatin was coadministered with fluconazole, a potent CYP2C9 inhibitor.
In vitro and in vivo data indicate that rosuvastatin has no clinically significant cytochrome P450 interactions (as substrate, inhibitor or inducer). Consequently, there is little potential for drug-drug interactions upon coadministration with agents that are metabolized by cytochrome P450.
Metabolism
Rosuvastatin undergoes limited metabolism in the liver
mainly by the cytochrome P450 isoenzyme CYP2C9
(approximately 10%).
Approximately 90% of the rosuvastatin dose is excreted
unchanged in the faeces (consisting of absorbed and
non-absorbed active substance) and the remaining part is
excreted in urine.
Properties of Rosuvastatin
| Melting point: | 161.9 °C |
| Boiling point: | 745.6±70.0 °C(Predicted) |
| Density | 1.368±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Solid |
| pka | 4.25±0.10(Predicted) |
| color | White to Pale Beige |
| CAS DataBase Reference | 287714-41-4(CAS DataBase Reference) |
| EPA Substance Registry System | 6-Heptenoic acid, 7-[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]-3,5-dihydroxy-, (3R,5S,6E)- (287714-41-4) |
Safety information for Rosuvastatin
Computed Descriptors for Rosuvastatin
Rosuvastatin manufacturer
Apex Healthcare Limited
Basic Pharma Life Science Pvt Ltd
Sainor Life Sciences Pvt Ltd
Dayaram Pharma Chem
Zenfold Sustainable Technologies (ZST)
Alcove International
Elixir Pharma
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
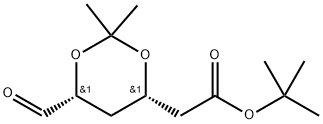
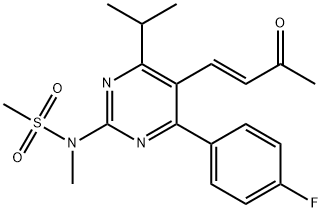
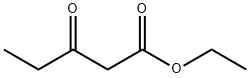
![Phosphonium, [[4-(4-fluorophenyl)-6-(1-methylethyl)-2-[methyl(methylsulfonyl)amino]-5-pyrimidinyl]me](https://img.chemicalbook.in/CAS/GIF/885477-83-8.gif)
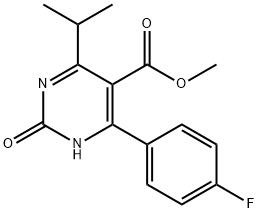
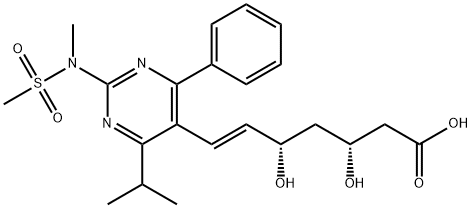
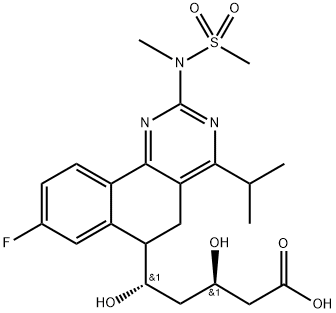
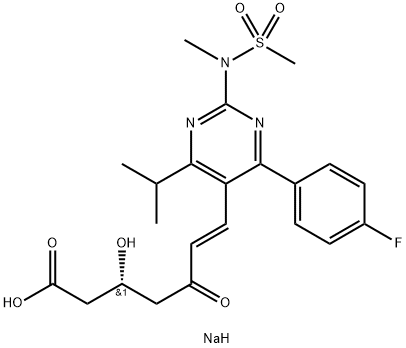
You may like
-
 287714-41-4 98%View Details
287714-41-4 98%View Details
287714-41-4 -
 ROSUVASTATIN 10.0% DC GRANULES 98%View Details
ROSUVASTATIN 10.0% DC GRANULES 98%View Details
287714-41-4 -
 ROSUVASTATIN 287714-41-4 98%View Details
ROSUVASTATIN 287714-41-4 98%View Details
287714-41-4 -
 Rosuvastatin 98%View Details
Rosuvastatin 98%View Details
287714-41-4 -
 287714-41-4 Rosuvastatin 98%View Details
287714-41-4 Rosuvastatin 98%View Details
287714-41-4 -
 Rosuvastatin 98%View Details
Rosuvastatin 98%View Details -
 287714-41-4 Rosuvastatin 98%View Details
287714-41-4 Rosuvastatin 98%View Details
287714-41-4 -
 287714-41-4 98%View Details
287714-41-4 98%View Details
287714-41-4