ROQUEFORTINE C
Synonym(s):2H-Pyrazino[1′,2′:1,5]pyrrolo[2,3-b]indole-1,4(3H,5aH)-dione, 10b-(1,1-dimethyl-2-propen-1-yl)-6,10b,11,11a-tetrahydro-3-(1H-imidazol-5-ylmethylene)-,(3E,5aS,10bR,11aS)-;Roquefortine from Penicillium roqueforti
- CAS NO.:58735-64-1
- Empirical Formula: C22H23N5O2
- Molecular Weight: 389.45
- MDL number: MFCD00133802
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
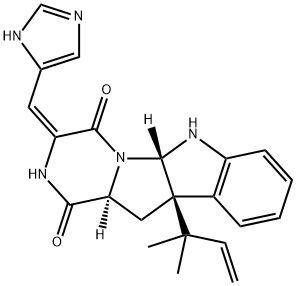
What is ROQUEFORTINE C?
Description
The third alkaloid isolated from a surface culture of Penicillium roqueforti, roquefortine C is present in only small quantities and the amount obtained is insufficient for the full structure to be established.
Description
Roquefortine C is a mycotoxin that was first isolated from a strain of P. roqueforti, a species of Penicillium commercially used to ripen blue-
The Uses of ROQUEFORTINE C
Roquefortine C is a potent tremorgenic mycotoxin originally isolated from Penicillium roqueforti in 1975 in Japan. Parallel research by Scott and colleagues lead to the structure elucidation of roquefortine C as an unusual diketopiperazine formed by coupling a prenylated tryptophan and histidine. Roquefortine C was subsequently found to be produced by a diverse range of fungi, most notably Penicillium species. Roquefortine is an important mycotoxin as low levels can be found in foodstuffs.
The Uses of ROQUEFORTINE C
Roquefortine C has been used as a standard for the quantification of roquefortine C by high-performance liquid chromatography (HPLC). It has also been used as a standard for the quantification of roquefortine C by liquid chromatography-mass spectrometry (LC?MS/MS).
What are the applications of Application
Roquefortine C is a potent neurotoxin produced most notably by Penicillium species
Definition
ChEBI: Roquefortine C is a pyrroloindole.
Biochem/physiol Actions
Roquefortine C is a paralytic neurotoxin of a dioxopiperazine structure produced by a diverse range of fungi, most notably Penicillium species. It has been found in blue cheese and in many other food products due to natural occurrence and contamination. Roquefortine C was found to be active on a wide range of organisms. It inhibits the growth of Gram-positive bacteria, and cockerels treated with roquefortine lost their righting reflex and died within 8-12 hours. Mice injected with roquefortine C experienced neurotoxic properties. Roquefortine C was also reported to inhibit cytochrome P450 as well as tubulin polymerization.
References
Ohmono et al., Agr. Bioi. Chem., 39, 1333 (1975)
Properties of ROQUEFORTINE C
| Melting point: | 202-205℃ |
| Boiling point: | 768.3±60.0 °C(Predicted) |
| Density | 1.37±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | chloroform: soluble1mg/mL |
| form | White to off-white solid. |
| pka | 11.10±0.40(Predicted) |
| color | White to off-white |
| Stability: | Hygroscopic |
Safety information for ROQUEFORTINE C
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for ROQUEFORTINE C
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
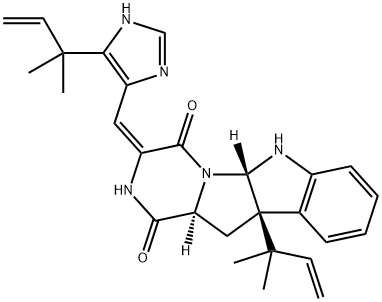
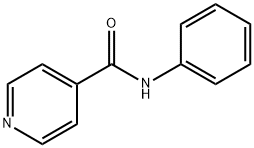


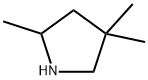
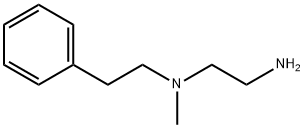
![N-[1-(aminomethyl)butyl]-N,N-dimethylamine](https://img.chemicalbook.in/CAS/GIF/19764-60-4.gif)
![hexahydropyrrolo[1,2-a]pyrazin-4(1H)-one](https://img.chemicalbook.in/CAS/20150408/GIF/1000577-63-8.gif)
You may like
-
 Roquefortine C CAS 58735-64-1View Details
Roquefortine C CAS 58735-64-1View Details
58735-64-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1