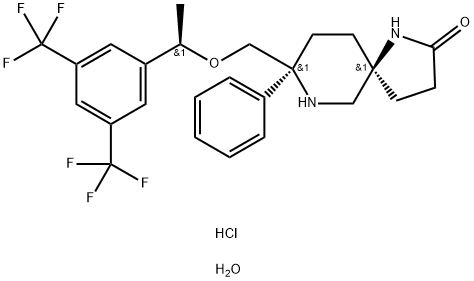
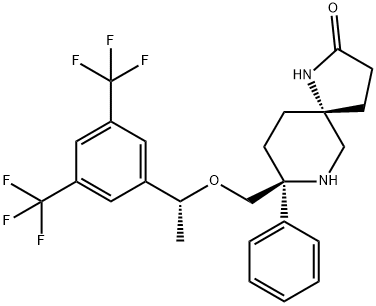
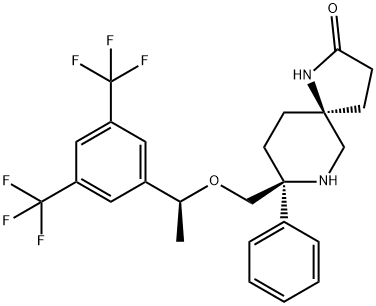
ROLAPITANT HYDROCHLORIDE
- CAS NO.:914462-92-3
- Empirical Formula: C25H29ClF6N2O3
- Molecular Weight: 554.96
- MDL number: MFCD23105917
- EINECS: 682-730-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-29 14:36:51
What is ROLAPITANT HYDROCHLORIDE?
Description
Rolapitant hydrochloride hydrate, originally discovered by Schering-Plough and later developed by TESARO, Inc., was approved by the FDA in September 2015 for the prevention of delayed chemotherapy-induced nausea and vomiting (CINV) in combination with other antiemetic agents. Rolapitant is a highly selective NK-1 receptor antagonist, exhibiting >1000- fold selectivity for NK-1 over human NK-2 and NK-3 receptors in vitro. In contrast to other NK-1 inhibitors that play an essential role in delayed CINV therapy, rolapitant shows no inhibition of CYP3A4, eliminating the need for concern when coadministering with CYP34A substrates. Additionally, rolapitant is an orally active agent with a relatively long half-life (180 h), providing potential opportunities for single- and prechemotherapy-based treatments. In three large clinical trials involving patients receiving moderately emetogenic chemotherapy (MEC) and highly emetogenic chemotherapy (HEC), subjects using rolapitant as a cotherapy with granisetron and dexamethasone showed a significant improvement in complete response compared to those receiving treatments of granisetron and dexamethasone.
The Uses of ROLAPITANT HYDROCHLORIDE
Rolapitant Hydrochloride Hydrate is a raw material for pharmaceutical formulations.
Definition
ChEBI: A hydrate that is the monohydrate form of rolapitant hydrochloride. Used for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy.
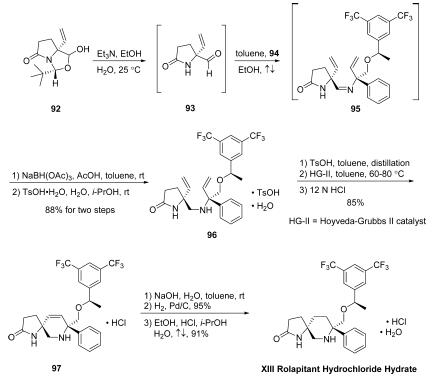
Synthesis
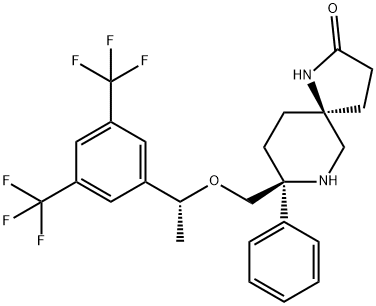
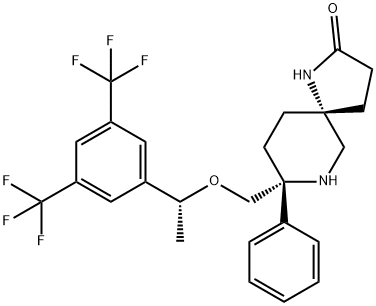
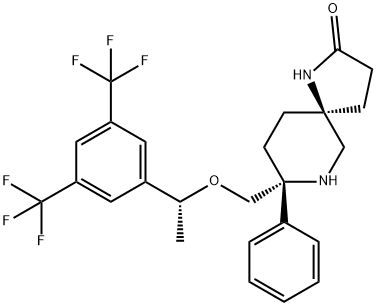
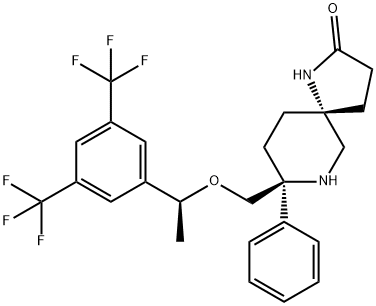
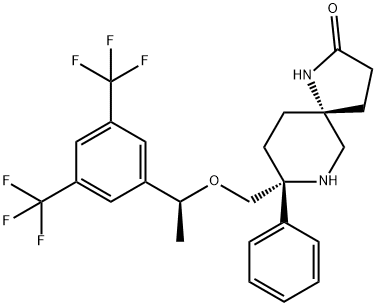
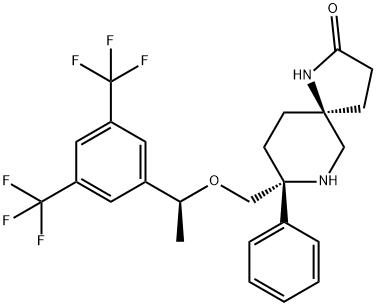
Rolapitant features a fascinating molecular architecture
consisting of two tetrasubstituted stereogenic carbon centers
situated at the 2- and 5-carbons within a central piperidine ring and a spirocyclic array residing at the 5-position and a phenyl
ring and ethereal linkage branching from the 2-position. The overall synthetic strategy to secure rolapitant
hydrochloride hydrate relies upon the union of two advanced
chiral building blocks that contain functional groups capable of
securing the central piperidine ring. These two key
intermediates, pyroglutamate derivative 93 and allylic amine
94, each bear one of the essential stereocenters embedded
within the structure of the active pharmaceutical ingredient.

The first of these advanced intermediates, amidoaldehyde 93, is
generated directly by base-mediated decomposition of
pyroglutamic aminal 92. Subjection of 92 to triethylamine in
EtOH/H2O at ambient temperatures led to generation of chiral
allyl aldehyde 93, which was not isolated but condensed
immediately with amine 94 in the presence of
refluxing toluene to provide divinyl imine 95, which underwent
immediate reduction using NaBH(OAc)3 in AcOH/toluene to
furnish the free amine. The free amine was converted to the
corresponding tosylate monohydrate salt and triturated,
providing 96 as a white crystalline powder after subjection to
TsOH?¤H2O in i-PrOH/H2O. Divinyl amine 96 could then be
reacted with a solution of TsOH in toluene, distilled, and
directly combined with a toluene solution of Hoveyda-Grubbs
second-generation catalyst (HG-II) under heating conditions,
leading to the desired ring-closing metathesis product 97 as the
HCl salt (85% yield over two steps) after filtration, distillation,
and workup with 12N HCl. Washing of a toluene solution of 97
with aqueous NaOH and subsequent treatment of the resulting
organic solution with H2, wet Pd/C, and additional granular
activated carbon (Nuchar Aquaguard) led to the fully reduced
piperidine product in high yield (95%). Rolapitant hydrochloride
hydrate XIII was accessed thereafter by precipitation
from a solution of EtOH/i-PrOH/H2O/HCl, providing the
product as a white solid (91% yield).
Properties of ROLAPITANT HYDROCHLORIDE
| Melting point: | >149oC (dec.) |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | White |
Safety information for ROLAPITANT HYDROCHLORIDE
Computed Descriptors for ROLAPITANT HYDROCHLORIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8