Ritalin
- CAS NO.:113-45-1
- Empirical Formula: C14H19NO2
- Molecular Weight: 233.31
- EINECS: 204-028-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:31
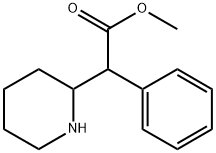
What is Ritalin?
Absorption
Concerta?: Methylphenidate is readily absorbed. Following oral administration of Concerta, plasma methylhphenidate concentrations reach an initial maximum at about 1 hour followed by gradual ascending concentrations over the next 5-9 hours. Mean times to reach peak plasma concentrations across all doses of Concerta occurred between 6-10 hours. Once daily dosing minimizes the fluctuations between peak and trough concentrations associated with multiple doses of immediate-release methylphenidate treatments. Depending on the doses provided, Cmax was found to range from 6.0-15.0ng/mL, Tmax ranged from 8.1-9.4h, and AUC ranged from 50.4-121.5 ng·h/mL in children.
When provided as Concerta?, methylphenidate is released through the patented Osmotic Controlled-Release Oral Delivery (OROS) system where 22% of the dose is provided as an immediate release and 78% is provided through a gradual release. OROS is comprised of an osmotically active trilayer core surrounded by a semipermeable membrane with an immediate-release drug overcoat. Within an aqueous environment, such as the stomach, the drug overcoat, which consists of 22% of the dose, dissolves within one hour, providing an initial immediate-release formulation of methylphenidate. Water then permeates through the membrane into the tablet core where the osmotically active polymer excipients expand, allowing methylphenidate to release slowly through the orifice over a period of 6-7 hours. Concerta also provides a sustained 10-12 hour effect, allowing for once-daily dosing.
Biphentin?: Methylphenidate is rapidly and extensively absorbed following oral administration, with peak blood levels obtained in 1-3 hours.
When provided as Biphentin?, methylphenidate is released through a multi-layer release delivery system (MLRTM) where 40% of the dose is provided as an immediate release and 60% is provided through a gradual release. Biphentin was designed to be an alternative to separate doses of immediate-release (IR) methylphenidate by providing a biphasic concentration-time profile when given as a single dose. The MLRTM release system allows for a sustained effect for 10-12 hours, allowing for once-daily dosing that covers the major times that ADHD impairment might occur (such as school, homework periods, during the workday, etc).
Methylphenidate (immediate release): Methylphenidate hydrochloride is rapidly and extensively absorbed from the tablets following oral administration; however, owing to extensive first-pass metabolism, bioavailability is low (approx. 30%) and large individual differences exist (11-52%). In one study, the administration of methylphenidate hydrochloride with food accelerated absorption but had no effect on the amount absorbed. Peak plasma concentrations of 10.8 and 7.8 ng/mL were observed, on average, 2 hours after administration of 0.30 mg/kg in children and adults, respectively. Peak plasma concentrations showed marked variability between subjects. Both the area under the concentration-time curve (AUC), and the peak plasma concentrations (Cmax) showed dose-proportionality.
Toxicity
Symptoms of overdose include vomiting, agitation, tremors, hyperreflexia, muscle twitching, convulsions (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, mydriasis, and dryness of mucous membranes. LD50=190mg/kg (orally in mice)
Originator
Ritalin,Ciba,US,1958
History
Methylphenidate is a piperidine derivative that is a central nervous system stimulant. Methylphenidate, in its hydrochloride form, is the active ingredient in the common medication Ritalin. Methylphenidate was first synthesized from benzyl cyanide and 2-chloropyridine in Basel, Switzerland in 1944 by Ciba chemist Leandro Panizzon (1907 ?).
Panizzon named the substance Ritaline after his wife, whose nickname was Rita. Panizzon and Max Hartmann proposed an improved synthesis for methylphenidate and obtained a U.S. patent for its preparation in 1950 (U.S. Patent Number 2507631). In 1954, methylphenidate was patented for use as an agent for treating psychological disorders under the name Ritalin by the Ciba pharmaceutical company.(Ciba subsequently merged with the Geigy company to become Ciba-Geigy, and then Ciba-Geigy merged with Sandoz to form Novartis.) Methylphenidate was first used to reverse drug-induced coma. Ritalin was approved by the Food and Drug Administration (FDA) in 1955 and introduced in the United States in 1956 for several conditions including depression, senile behavior, lethargy, and narcolepsy.
Hyperactive children were first treated with stimulants in 1937. Since methylphenidate first appeared on the market, its use for treating ADHD has steadily increased. It is the most commonly used medication for treating children diagnosed with ADHD.
The Uses of Ritalin
Stimulant.
The Uses of Ritalin
Methylphenidate's mode of action is not completely known, but it is believed that ADHD symptoms are related to the dopaminergic areas of the brain. Animal studies indicate that methylphenidate affects several neurotransmitters to counteract ADHD behavior. Methylphenidate binds to dopamine transporters in the presynaptic neuron, blocking the reuptake of dopamine and increasing extracellular dopamine. Methylphenidate also infl uences norepinephrine reuptake and infl uences serotonin to a minor degree.
In addition to ADHD, methylphenidate is used for several other medical conditions.It continues to be used for narcolepsy. It has also been used in treating depression, especially in elderly populations. Methylphenidate has been suggested for use in the treatment of brain injury from stroke or brain trauma; it has also been suggested to improve appetite and the mood of cancer and HIV patients. Another use is for pain control and/or sedation for patients using opiates.
Health concerns are associated with the use of methylphenidate.Some of the commonly reported adverse effects associated with its use are insomnia, nervousness, hypertension, headache, anorexia, and tachycardia;less common effects include weight loss, nausea, and angina. Studies have indicated that methylphenidate lead to liver tumors in mice, but limited studies on its carcinogenicity in animals have not led the FDA to change recommendations on its use. Because methylphenidate is a stimulant and readily available, it has a potential for drug abuse.
Indications
Methylphenidate is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years of age and older and for the treatment of narcolepsy.
Background
Methylphenidate is a central nervous system stimulant used most commonly in the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) and for narcolepsy. Also known as the marketed products Ritalin, Concerta, or Biphentin, methylphenidate is used with other treatment modalities (psychological, educational, cognitive behaviour therapy, etc) to improve the following group of developmentally inappropriate symptoms associated with ADHD: moderate-to-severe distractibility, short attention span, hyperactivity, emotional lability, and impulsivity. Long-acting formulations of psychostimulants such as methylphenidate, Dextroamphetamine, and Lisdexamfetamine are considered the most effective and widely used treatment for ADHD, and are considered first-line options for children, adolescents, and adults as recommended by CADDRA (Canadian ADHD Resource Alliance). CADDRA recommends the use of methylphenidate due to long term studies, of over twenty years in duration, which show methylphenidate is safe and effective.
While its exact mechanism is unclear, methylphenidate (MPH) has been shown to act as a norepinephrine and dopamine reuptake inhibitor (NDRI), thereby increasing the presence of these neurotransmitters in the extraneuronal space and prolonging their action. There is a dose-related effect of psychostimulants on receptor stimulation, where higher doses are shown to increase norepinephrine (NE) and dopamine (DA) efflux throughout the brain which can result in impaired cognition and locomotor-activating effects. In contrast, low doses are found to selectively activate NE and DA neurotransmission within the prefrontal cortex which is an area of the brain thought to play a prominent role in ADHD pathophysiology, thereby improving clinical efficacy and preventing side effects. The lower doses used to treat ADHD are not associated with the locomotor-activating effects associated with higher doses and instead reduce movement, impulsivity, and increase cognitive function including sustained attention and working memory. Methylphenidate's beneficial effects in sustaining attention have also been shown to be mediated by alpha-1 adrenergic receptor activity. Clinical findings have shown that children with ADHD have an abnormality in the dopamine transporter gene (DAT1), the D4 receptor gene (DRD-4), and/or the D2 receptor gene that may be at least partly overcome by the dopaminergic effects of methylphenidate, suggesting a possible mode of action.
When provided as Biphentin?, methylphenidate is released through a multi-layer release delivery system (MLRTM) where 40% of the dose is provided as an immediate release and 60% is provided through a gradual release. Biphentin was designed to be an alternative to separate doses of immediate-release (IR) methylphenidate by providing a biphasic concentration-time profile when given as a single dose. The MLRTM release system allows for a sustained effect for 10-12 hours, allowing for once-daily dosing that covers the major times that ADHD impairment might occur (such as school, homework periods, during the work day, etc).
When provided as Concerta?, methylphenidate is released through the patented Osmotic Controlled-Release Oral Delivery (OROS) system where 22% of the dose is provided as an immediate release and 78% is provided through a gradual release. OROS is comprised of an osmotically active trilayer core surrounded by a semipermeable membrane with an immediate-release drug overcoat. Within an aqueous environment, such as the stomach, the drug overcoat, which consists of 22% of the dose, dissolves within one hour, providing an initial immediate-release formulation of methylphenidate. Water then permeates through the membrane into the tablet core where the osmotically active polymer excipients expand, allowing methylphenidate to release slowly through the orifice over a period of 6-7 hours. Concerta also provides a sustained 10-12 hour effect, allowing for once-daily dosing.
Methylphenidate contains a blackbox warning stating that CNS stimulants, including methylphenidate-containing products and amphetamines, have a high potential for abuse and dependence. This abuse potential is likely related to the effects associated with higher doses of methylphenidate, which induce surface expression of the dopamine transporter (DAT). In particular, increased dopamine in key brain areas is associated with the reinforcing and addictive properties of psychostimulants such as methylphenidate, and even amplifies the potency and reinforcing effects of other drugs of abuse such as amphetamines, making ADHD sufferers more susceptible to their addictive effects. Concerns about abuse potential have spurred research into medications with fewer effects on DAT and the use of non-stimulant ADHD medications including Atomoxetine and Guanfacine.
Definition
ChEBI: Methyl phenyl(piperidin-2-yl)acetate is a amino acid ester that is methyl phenylacetate in which one of the hydrogens alpha to the carbonyl group is replaced by a piperidin-2-yl group. It is a member of piperidines, a methyl ester and a beta-amino acid ester.
Manufacturing Process
As described in US Patent 2,507,631, 80 g of pulverized sodium amide are gradually added, while stirring and cooling, to a solution of 117 g of phenylacetonitrile and 113 g of 2-chloropyridine in 400 cc of absolute toluene. The mixture is then slowly heated to 110° to 120°C and maintained at this temperature for 1 hour. Water is added thereto after cooling, the toluene solution is shaken with dilute hydrochloric acid and the hydrochloric acid extracts are made alkaline with concentrated caustic soda solution. A solid mass is separated thereby which is taken up in acetic ester and distilled, αphenyl-α-pyridyl-(2)-acetonitrile passing over at 150° to 155°C under 0.5 mm pressure. When recrystallized from ethyl acetate it melts at 88° to 89°C, the
yield amounting to 135 g.
100 g of α-phenyl-α-pyridyl-(2)-acetonitrile are introduced into 400 cc of
concentrated sulfuric acid, allowed to stand overnight at room temperature,
poured into ice and rendered alkaline with sodium carbonate. α-Phenyl-αpyridyl-(2)-acetamide is precipitated thereby which melts at 134°C after
recrystallization from ethyl acetate.
100g of the resulting α-phenyl-α-pyridyl-(2)-acetamide, when dissolved in one
liter of methyl alcohol and treated for 6 hours at water-bath temperature with
hydrogen chloride, and after concentrating, diluting with water and rendering
alkaline with sodium carbonate, yield 90 g of the α-phenyl-α-pyridyl-(2)-acetic
acid methylester of MP 74° to 75°C (from alcohol of 50% strength).
The α-phenyl-α-piperidyl-(2)-acetic acid methylester of BP 135° to 137°C
under 0.6 mm pressure is obtained in theoretical yield by hydrogenation of 50
g of α-phenyl-α-pyridyl(2)-acetic acid methylester in glacial acetic acid in the
presence of 1 g of platinum catalyst at room temperature, while taking up 6
hydrogen atoms. Reaction with HCl gives the hydrochloride. Resolution of
stereoisomers is described in US Patent 2,957,880.
brand name
Daytrana(Shire);4311 ciba;Centedrin;Cetedrin;Methidate;Ritalin sr;Ritaline.
Therapeutic Function
Psychostimulant
World Health Organization (WHO)
Methylphenidate, a piperidine derivative with mild central stimulant activity, was introduced in 1956. Its pharmacological properties resemble those of amfetamines and it shares their abuse potential. Methylphenidate retains a place as an adjunct in the treatment of hyperkinetic syndromes in both children and adults. It is controlled under Schedule II of the 1971 Convention on Psychotropic Substances. (Reference: (UNCPS2) United Nations Convention on Psychotropic Substances (II), , , 1971)
Pharmacokinetics
Methylphenidate is a racemic mixture comprised of the d- and l-isomers. The d-isomer is more pharmacologically active than the l-isomer. Radioligand binding studies demonstrate that binding of methylphenidate in the brain is localized to dopamine-rich areas, in particular in the prefrontal cortex which has been demonstrated to play a prominent role in ADHD pathophysiology. In a number of animal models, methylphenidate enhances locomotor activity and induces stereotypic behaviours.
Safety Profile
Poison experimentally by ingestion, intraperitoneal, intravenous, and subcutaneous routes. Moderately toxic to humans by intravenous route. Human systemic effects by intravenous route: dyspnea. An experimental teratogen. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
Methylphenidate is hepatically metabolized. More specifically, it is rapidly and extensively metabolized by carboxylesterase CES1A1. Via this enzyme, methylphenidate undergoes de-esterification to ritalinic acid (a-phenyl-2-piperidine acetic acid, PPAA), which has little to no pharmacologic activity.
Properties of Ritalin
| Melting point: | 74.5°C |
| Boiling point: | 327℃ |
| Density | 1.070 |
| refractive index | 1.5110 (estimate) |
| Flash point: | 152℃ |
| pka | 9.51±0.10(Predicted) |
| CAS DataBase Reference | 113-45-1 |
| EPA Substance Registry System | 2-Piperidineacetic acid, ?-phenyl-, methyl ester (113-45-1) |
Safety information for Ritalin
Computed Descriptors for Ritalin
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran





You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4