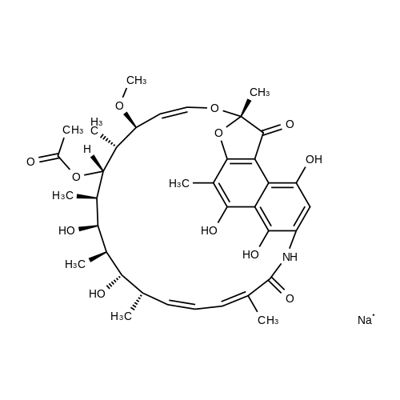
Rifapentine
Synonym(s):DL 473
- CAS NO.:61379-65-5
- Empirical Formula: C47H64N4O12
- Molecular Weight: 877.03
- MDL number: MFCD00866806
- EINECS: 262-743-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 15:18:15
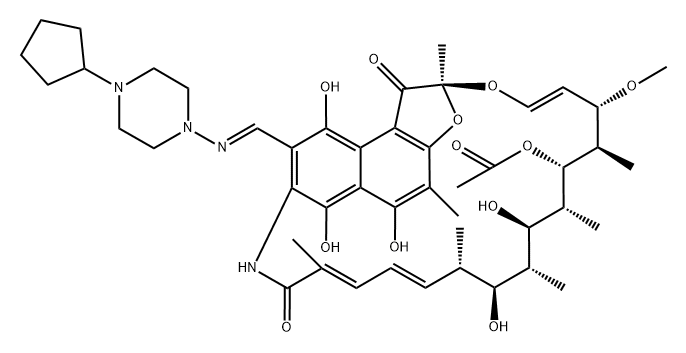
What is Rifapentine?
Absorption
Rapidly and well absorbed from the gastrointestinal tract.
Description
Rifapentine is a broad spectrum antibiotic highly active against Gram-positive bacteria and Neisseria gonorrhoea. It is reported to be 10 times more active than the structurally related rifampicin against Mycobacrerim fuberculosis. Unlike rifampicin the bioavailability of rifapentine is significantly increased when administered after a meal.
Chemical properties
Crystalline Solid
Originator
Shanghai No. 5 Factory (China)
The Uses of Rifapentine
Semi-synthetic rifamycin. Antibacterial (tuberculostatic)
The Uses of Rifapentine
antibacterial (tuberculostatic);'inhibits DNA-dependent RNA polymerase in susceptible strains of Mycobacterium tuberculosis
The Uses of Rifapentine
PDE5 inhibitor for erectile dysfunction A semisynthetic ansamycin antibiotic, cyclopentyl derivative of Rifamycin. Treatment of pulmonary tuberculosis
Indications
For the treatment of pulmonary tuberculosis.
Background
Rifapentine is an antibiotic drug used in the treatment of tuberculosis. It inhibits DNA-dependent RNA polymerase activity in susceptible cells. Specifically, it interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme.
What are the applications of Application
Rifapentine is an antibiotic
Indications
Rifapentine is an analogue of rifampin that is active against M. tuberculosis and M. avium. Rifapentine’s mechanism of action, cross-resistance, hepatic induction of P450 enzymes, drug interactions, and toxic profile are similar to those of rifampin. It has been used in the treatment of tuberculosis caused by rifampinsusceptible strains.
Definition
ChEBI: Rifapentine is a N-alkylpiperazine, a N-iminopiperazine and a member of rifamycins. It has a role as an antitubercular agent and a leprostatic drug.
brand name
Priftin (Sanofi Aventis);Rifampin.
Antimicrobial activity
Activity is similar to that of rifampicin, but it is more active against atypical mycobacteria, especially the M. avium complex (MIC <0.06–0.5 mg/L). It has good activity on staphylococci and streptococci (MIC 0.01–0.5 mg/L), L. monocytogenes and Brucella spp.; less against Enterococcus faecalis (MIC 1–4 mg/L). Bacteroides spp. are inhibited by 0.5–2 mg/L. Gram-negative cocci are susceptible and, although some Gram-negative bacilli are inhibited by 4–32 mg/L, most are resistant.
Hazard
Moderately toxic by ingestion.
Pharmaceutical Applications
An analog of rifampicin in which a cyclopentyl group is substituted for a methyl group on the piperazine ring. It is available for oral administration.
Mechanism of action
Because relapse and the emergence of resistant strains of bacteria are associated with poor patient compliance, reduced dosing is expected to increase compliance. Initial clinical studies actually showed that the relapse rates in patients treated with rifapentine (10%) were higher than those in the patients treated with RIF (5%). It was found that poor compliance with the nonrifamycin antituberculin agents was responsible for the increased relapse.
Pharmacokinetics
Rifapentine is an antibiotic that inhibits DNA-dependent RNA polymerase activity in susceptible cells. Specifically, it interacts with bacterial RNA polymerase but does not inhibit the mammalian enzyme. It is bactericidal and has a very broad spectrum of activity against most gram-positive and gram-negative organisms (including Pseudomonas aeruginosa) and specifically Mycobacterium tuberculosis. Because of rapid emergence of resistant bacteria, use is restricted to treatment of mycobacterial infections and a few other indications. Rifampin is well absorbed when taken orally and is distributed widely in body tissues and fluids, including the CSF. It is metabolized in the liver and eliminated in bile and, to a much lesser extent, in urine, but dose adjustments are unnecessary with renal insufficiency.
Pharmacokinetics
Oral absorption:c. 70%
Cmax600 mg oral :12 mg/L after 5 h
Plasma half-life:13 h
Volume of distribution:1.5 L/kg
Plasma protein binding:97%
absorption
The absolute oral bioavailability of rifapentine has not been determined. The relative bioavailability of capsules (with an oral solution as reference) is 70%. Food increases absorption: a 600 mg dose taken after a meal gives Cmax and AUC values 44% higher than under fasting conditions. The extended halflife provides therapeutic concentrations for at least 72 h after administration, allowing less frequent dosing.
Distribution
Animal data suggest that it is well distributed in the body, with tissue concentrations exceeding the plasma concentration, except in bone, testes and brain. The ratio of intracellular:extracellular concentration in macrophages was estimated as 24:1.
Metabolism
The main metabolite is an antimicrobially active 25-desacetyl derivative. Although it induces liver cytochromes it is not an inducer of its own metabolism, which is mediated by an esterase. The peak concentration of 25-desacetyl rifapentine is about one-third of that of the unchanged drug, and is attained after about 11 h.
excretion
The main route of elimination is through the bile. In healthy volunteers about 70% of a 600 mg dose of 14C rifapentine was recovered in the feces, and less than 17% in the urine. There is evidence of enterohepatic recycling in humans.
Clinical Use
Tuberculosis (in combination with other antituberculosis drugs)
Side Effects
Signs of teratogenic effects and fetal toxicity have been observed when administered during pregnancy to rats and rabbits. Rifapentine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The most common adverse effect observed in combinations with other antimycobacterial agents was hyperuricemia, most probably due to pyrazinamide. Effects likely to be due to rifapentine were neutropenia (3.7% of patients) and hepatitis (increased transaminases in 1.6% of patients).
in vitro
the activities of rifampin and rifapentine against mycobacterium tuberculosis residing in human monocytederived macrophages were determined. the mic and mbc of rifapentine for intracellular bacteria were two- to four-fold lower than those of rifampin. for extracellular bacteria, this difference was less noticeable. [1].
in vivo
once-a-week exposure to rifapentine concentrations equivalent to that attained in blood after one 600-mg dose resulted during the first week in a dramatic decline in the number of bacteria, and such decline was maintained at a minimal level for a period of four weeks. the prolonged effect of rifapentine found may be associated with high ratios of intracellular accumulation, which were four- to fivefold higher than those found for rifampin [1].
Metabolism
Hepatic
References
[1] mor n, simon b, mezo n, heifets l. comparison of activities of rifapentine and rifampin against mycobacterium tuberculosis residing in human macrophages. antimicrob agents chemother. 1995 sep;39(9):2073-7.
[2] temple me, nahata mc. rifapentine: its role in the treatment of tuberculosis. ann pharmacother. 1999 nov;33(11):1203-10.
Properties of Rifapentine
| Melting point: | 179-1800C |
| Boiling point: | 969.3±65.0 °C(Predicted) |
| Density | 1.35±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | methanol: soluble2mg/mL, clear, red to red-brown |
| form | powder |
| pka | 4.81±0.70(Predicted) |
| color | Red |
Safety information for Rifapentine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Rifapentine
Abamectin manufacturer
AVD pharmaceuticals Pvt Ltd
New Products
4-Fluorophenylacetic acid (S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid 5-Aminoimidazole-4-Carbonitrile 4-chloro-3,5-dinitropyridine 2'-Methoxy-biphenyl-2-carboxaldehyde 2-(2-Aminoethyl)isothiourea dihydrobromide, 1-(4-chlorophenyl)propan-1-one 2-Ethyl-4-methyl-1-pentanol DIISOPROPYL MALONATE PENTAFLUOROPHENOL 2-Aminonicotinic acid 6-(4-AMINOPHENYL)-5-METHYL-4,5-DIHYDRO-3(2 H)-PYRDAZINONE β-BUTYROLACTONE 3-OXO-CYCLOBUTANECARBOXYLIC ACID 3-methyl xanthine 1H-Pyrazole-3-carboxylic acid [1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3- (trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester 2H-Cyclopenta[b]furan-2,5-diol, hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1- buten-1-yl]-, (3aR,4R,5R,6aS)- 2,5-Dibromopyridine Dimethyl (2-oxo-4-phenylbutyl)phosphonate S-(2-Chloro-3-nitrophenyl) O-ethyl carbonodithioate 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carboxylic acid 2,4-Dichloro-1-[2-nitro-4-(trifluoromethyl)phenoxy]benzeneRelated products of tetrahydrofuran




You may like
-
 61379-65-5 Rifapentine 98%View Details
61379-65-5 Rifapentine 98%View Details
61379-65-5 -
 Rifapentine CAS 61379-65-5View Details
Rifapentine CAS 61379-65-5View Details
61379-65-5 -
 Rifapentine, ≥98% (HPLC) CAS 61379-65-5View Details
Rifapentine, ≥98% (HPLC) CAS 61379-65-5View Details
61379-65-5 -
 Rifapentine 90.00% CAS 61379-65-5View Details
Rifapentine 90.00% CAS 61379-65-5View Details
61379-65-5 -
 Rifapentine CAS 61379-65-5View Details
Rifapentine CAS 61379-65-5View Details
61379-65-5 -
 (3R,4R)-1-benzyl-N,4- dimethylpiperidin-3-amine 477600-70-7 99%View Details
(3R,4R)-1-benzyl-N,4- dimethylpiperidin-3-amine 477600-70-7 99%View Details
477600-70-7 -
 Ortho Phenylene Diamine (OPDA) 95-54-5 99%View Details
Ortho Phenylene Diamine (OPDA) 95-54-5 99%View Details
95-54-5 -
 77-78-1 99%View Details
77-78-1 99%View Details
77-78-1