Rabeprazole Sodium
Synonym(s):2-([4-(3-Methoxypropoxy)-3-methylpyridin-2-yl]methylsulfinyl)-1H-benzo[d]imidazole;Pariprazole;Rabeprazole sodium
- CAS NO.:117976-90-6
- Empirical Formula: C18H21N3O3S.Na
- Molecular Weight: 382.43
- MDL number: MFCD02092688
- EINECS: 629-730-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
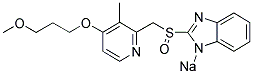
What is Rabeprazole Sodium?
Description
Rebeprazole sodium, marketed as Pariet in Japan, was initially launched for the treatment of peptic ulcers, including both gastric and duodenal ulcers. It is synthesized through a six-step process starting from 4-chloro-2,3-dimethylpyridine N-oxide, which allows the formation of its basic skeleton through successive condensations.
Description
Rabeprazole is a proton pump inhibitor with the ability to selectively and irreversibly inhibit the gastric H+/K+ ATPase enzyme, showing an IC50 value of 72 nM. It demonstrates a faster activation and a broader pH range of effectiveness compared to other proton pump inhibitors such as omeprazole, lansoprazole, and pantoprazole.
In studies, rabeprazole has been shown to inhibit gastric acid secretion in animal models, specifically in pylorus-ligated rats and a rat model of gastric fistula, when administered at a dosage of 30 mg/kg. Additionally, it has been found to inhibit the growth of several strains of Helicobacter pylori in vitro, with MIC50 values ranging from 1.57 to 3.13 μg/mL.
Formulations containing rabeprazole are utilized in the treatment of various conditions, including ulcers, pathological hypersecretory conditions, and gastroesophageal reflux disease (GERD).
Chemical properties
Rebeprazole sodium is White Crystalline Solid
Originator
Eisai (Japan)
The Uses of Rabeprazole Sodium
Rebeprazole sodium is a partially reversible gastric proton pump inhibitor
The Uses of Rabeprazole Sodium
anthelminthic, antiseptic, expectorant
The Uses of Rabeprazole Sodium
antibacterial and antifungal agent effective against gram-positive and gram-negative bacteria, yeast and fungi
What are the applications of Application
Rabeprazole Sodium Salt is a proton pump inhibitor
Definition
ChEBI: Rabeprazole sodium is an organic sodium salt. It contains a rabeprazole(1-).
brand name
Aciphex (Eisai Medical Research) .
General Description
Rabeprazole sodium, 2[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole sodium salt (Aciphex), is a white toslightly yellowish white solid. It is very soluble in water andmethanol, freely soluble in ethanol, chloroform, and ethyl acetate,and insoluble in ether and hexane. Rabeprazole is aweak base (pyridine N, pKa 4.53) and a weak acid (benzimidazoleN-H, pKa 0.62), faciliting sodium salt formation.
Rabeprazole sodium is formulated as enteric-coated,delayed-release tablets to allow the drug to pass throughthe stomach relatively intact. After oral administration of20-mg peak plasma concentrations (Cmax) occur over arange of 2 to 5 hours (Tmax). Absolute bioavailability for a20-mg oral tablet of rabeprazole (vs. IV administration) isapproximately 52%. The plasma half-life ofrabeprazole ranges from 1 to 2 hours. The effects of foodon the absorption of rabeprazole have not been evaluated.Rabeprazole is 96% bound to human plasma proteins.Rabeprazole is extensively metabolized in the liver. Thethioether and sulfone are the primary metabolites measuredin human plasma resulting from CYP3A oxidation.Additionally, desmethyl rabeprazole is formed via the actionof CYP2C19. Approximately 90% of the drug is eliminatedin the urine, primarily as thioether carboxylic acidand its glucuronide and mercapturic acid metabolites. Theremainder of the dose is recovered in the feces. No unchangedrabeprazole is excreted in the urine or feces.
Biochem/physiol Actions
Rabeprazole sodium is gastric proton pump inhibitor. It suppresses the production of acid in the stomach by inhibiting the gastric H+/K+ATPase (hydrogen-potassium adenosine triphosphatase) at the secretory surface of the gastric parietal cell. Rabeprazole sodium has been used clinically to treat acid-reflux disorders (GERD), peptic ulcer disease, H. pylori eradication, and prevent gastroinetestinal bleeds associated with NSAID use.
Drug interactions
Potentially hazardous interactions with other drugs
Antifungals: absorption of itraconazole and
ketoconazole reduced; avoid with posaconazole.
Antivirals: concentration of atazanavir and rilpivirine
reduced - avoid; concentration of raltegravir and
saquinavir possibly increased - avoid.
Clopidogrel: possibly reduced antiplatelet effect.
Cytotoxics: possibly reduced excretion of
methotrexate; avoid with dasatinib, erlotinib and
vandetanib; possibly reduced lapatinib absorption;
possibly reduced absorption of pazopanib.
Ulipristal: reduced contraceptive effect, avoid with
high dose ulipristal.
Metabolism
Rabeprazole is mainly metabolised via nonenzymatic reduction and, to a lesser extent, via the cytochrome P450 isoenzymes CYP2C19 and CYP3A4. Metabolites are excreted principally in the urine (about 90%) with the remainder in the faeces.
Properties of Rabeprazole Sodium
| Melting point: | 140-141°C dec. |
| storage temp. | -20°C |
| solubility | H2O: soluble10mg/mL (clear solution) |
| form | powder |
| color | white to beige |
| Merck | 14,8089 |
| Stability: | Hygroscopic |
Safety information for Rabeprazole Sodium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Rabeprazole Sodium
| InChIKey | KRCQSTCYZUOBHN-UHFFFAOYSA-N |
Rabeprazole Sodium manufacturer
Synaptics Labs Private Limited
Chemeca Drugs Private Limited (Vegesna Laboratories Pvt Ltd)
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



![1-[[4-(3-Methoxypropoxy)-3-methyl-2-pyridinyl]methyl]-2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]methyl]sulfinyl]-1H-benzimidazole](https://img.chemicalbook.in/CAS/GIF/935260-92-7.gif)
You may like
-
 117976-90-6 98%View Details
117976-90-6 98%View Details
117976-90-6 -
 RABEPRAZOLE SODIUM 98%View Details
RABEPRAZOLE SODIUM 98%View Details -
 RABEPRAZOLE SODIUM 95-99 %View Details
RABEPRAZOLE SODIUM 95-99 %View Details
117976-90-6 -
 Dexrabeprazole Sodium 98%View Details
Dexrabeprazole Sodium 98%View Details -
 Rabeprazole sodium 98%View Details
Rabeprazole sodium 98%View Details -
 Rabeprazole sodium 98%View Details
Rabeprazole sodium 98%View Details -
 Rabeprazole sodium >98% (HPLC) CAS 117976-90-6View Details
Rabeprazole sodium >98% (HPLC) CAS 117976-90-6View Details
117976-90-6 -
 Rabeprazole Sodium Salt CAS 117976-90-6View Details
Rabeprazole Sodium Salt CAS 117976-90-6View Details
117976-90-6