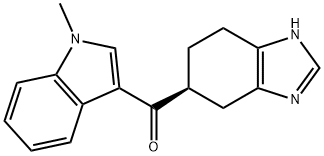
Ramosetron hydrochloride
Synonym(s):(R)-5-[(1-Methyl-3-indolyl)carbonyl]-4,5,6,7-tetrahydro-1H-benzimidazole hydrochloride;YM060;YM-060
- CAS NO.:132907-72-3
- Empirical Formula: C17H18ClN3O
- Molecular Weight: 315.8
- MDL number: MFCD00894748
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
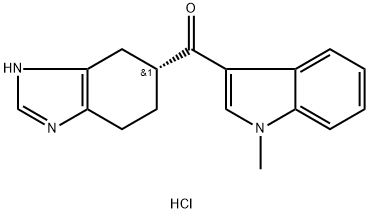
What is Ramosetron hydrochloride ?
Description
Ramosetron Hydrochloride is the only currently available drug indicated for the treatment of male patients with IBS-D in Japan. Ramosetron Hydrochloride was approved in July 1996 under a brand name of Nasea Injection 0.3 mg and in June 1998 under a brand name of Nasea OD tablets 0.1 mg for the treatment of gastrointestinal symptoms (nausea and vomiting) associated with therapy with antineoplastic drugs (e.g., cisplatin).
Nasea was launched in Japan for chemotherapy-induced emesis. Nasea was prepared by a four step sequence via the Vilsmeier-type coupling of 1-methylindole and 5-( 1- pyrrolidoncarbonyl)-4,5,6,7-tetrahydro-Hl-benzimidazole hydrochloride. The antiemetic activity arises because it is a potent 5-HT3 receptor antagonist that is i.v. and orally active. The (R)-isomer was found to be 100 times more potent than the (S)-isomer. It was able to inhibit cisplatin-induce emesis and was 8670 times more potent than netoclopramide.
Chemical properties
White Solid
Originator
Yamanouchi (Japan)
The Uses of Ramosetron hydrochloride
Ramosetron Hydrochloride is a selective serotonin 5-HT3 receptor antagonist and has structurally different from Ondansetron (O655000), Granisetron (G780000). It controls excessive bowel movement and diarrhea by inhibiting 5-HT3 receptors in the intestinal tract. Ramosetron Hydrochloride was approved as film-coated tablets in July 2008 and as orally disintegrating tablets in August 2013 for the indication of treatment of male patients with diarrhea-predominant irritable bowel syndrome.
Definition
ChEBI: Ramosetron hydrochloride is a member of indoles.
brand name
Nasea
Biochem/physiol Actions
Ramosetron is a tetra hydrobenzimidazole derivative, containing an indole ring. It inhibits colon contraction mediated by serotonin, competitively. this pharmacologic property confers the drug an application in improving diarrhea-predominant inflammatory bowel syndrome. Ramosetron hydrochloride is used for PONV prophylaxis and treatment. However, ramosetron is currently only licenced for use in Japan and selected Southeast Asian countries.
Properties of Ramosetron hydrochloride
| Melting point: | 244-246°C |
| alpha | D -42.9° (c 1.02 in methanol) |
| storage temp. | -20°C |
| solubility | H2O: soluble20mg/mL, clear |
| form | powder |
| color | white to beige |
| optical activity | [α]/D -40 to -45°, c = 1 in methanol |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 132907-72-3(CAS DataBase Reference) |
Safety information for Ramosetron hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Ramosetron hydrochloride
| InChIKey | XIXYTCLDXQRHJO-RFVHGSKJSA-N |
Ramosetron hydrochloride manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![5-(1-methyl-1H-indole-3-carbonyl)-1H-benzo[d]imidazol-2(3H)-one](https://img.chemicalbook.in/CAS/GIF/171967-71-8.gif)
You may like
-
 Ramosetron hydrochloride CAS 132907-72-3View Details
Ramosetron hydrochloride CAS 132907-72-3View Details
132907-72-3 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1