Quinidine
- CAS NO.:56-54-2
- Empirical Formula: C20H24N2O2
- Molecular Weight: 324.42
- MDL number: MFCD00135581
- EINECS: 200-279-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
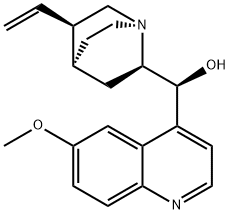
What is Quinidine?
Absorption
The absolute bioavailability of quinidine sulfate is approximately 70%, but it ranges from 45% to 100%. The less-than-complete quinidine sulfate bioavailability is a result of first-pass metabolism in the liver. In contrast, the absolute bioavailability of quinidine gluconate ranges from 70% to 80%, and relative to quinidine sulfate, quinidine from quinidine gluconate has a bioavailability of 1.03. The tmax of quinidine sulfate extended-release tablets is approximately 6 h, while the tmax of quinidine gluconate goes from 3 to 5 h. The peak serum concentration reached with immediate-release quinidine sulfate is delayed for about an hour when taken with food. Furthermore, the ingestion of grapefruit juice may decrease the rate of absorption of quinidine.
Toxicity
Quinidine overdoses have been well described. The ingestion of 5 g of quinidine resulted in the death of a toddler, while an adolescent was reported to survive after ingesting 8 g of quinidine. A 16-month that ingested quinidine tablets developed a concretion of bezoar in the stomach, which led to non-declining toxic levels of quinidine. A gastric aspirate revealed that quinidine levels were 50 times higher than the ones detected in plasma. In cases of massive overdose, it may be appropriate to perform an endoscopy. Acute quinidine overdoses are characterized by ventricular arrhythmias and hypotension. Other signs and symptoms of quinidine overdose may include vomiting, diarrhea, tinnitus, high-frequency hearing loss, vertigo, blurred vision, diplopia, photophobia, headache, confusion and delirium.
Description
Quinidine is a commonly used class I antiarrhythmic drug. It is a stereoisomer of quinine, originally derived from the bark of the cinchona tree. It exerts its antiarrhythmic effects on the heart by interacting with the electrophysiology mechanisms that cause arrhythmias to modify the abnormalities in impulse initiation and conduction. Quinidine depresses normal automaticity in cardiac fibers that may act as ectopic pacemakers causing arrhythmias. Quinidine also blocks the slowly inactivating, tetrodotoxin-sensitive Na current, the slow inward calcium current (ICa), the rapid (IKr) and slow (IKs) components of the delayed potassium rectifier current, the inward potassium rectifier current (IKI), the ATP-sensitive potassium channel (IKATP) and Ito.
Description
Quinidine is a stereoisomer of the antimalarial agent quinine and a class Ia antiarrhythmic agent. Quinidine blocks the voltage-gated sodium (Nav) channel Nav1.5 in a use-dependent manner. It decreases the amplitude and duration of action potentials in isolated canine ventricular myocytes. It inhibits KKr, peak INa, and late INa (IC50s = 4.5, 11, and 12 μM, respectively) and can induce torsade de pointes in isolated rabbit hearts when used at a concentration of 1 μM. Quinidine induces QT prolongation in dogs. It also binds to M2 muscarinic acetylcholine receptors (Ki = 7.5 μM for human recombinant receptors expressed in HM2-B10 cells). Formulations containing quinidine have been used in the treatment of atrial fibrillation and ventricular arrhythmias.
Chemical properties
white to light yellow crystal powde
Physical properties
Appearance: Quinidine is commonly used in its sulfate form with white needle-like crystal and bitter smell. It changes color easily when exposed to light. Solubility: It was soluble in ethanol and chloroform. Its water solubility is 0.05?g/100?mL (20?°C). Specific optical rotation: 256° (c?=?1, EtOH). Melting point: 168–172?°C.
History
In 1820, the French chemists Pierre Pelletier and Joseph Caventou extracted some alkaloids from the cinchona bark, including quinine and quinidine. Subsequently, quinine was demonstrated to play a very important role in the treatment of malaria after a number of scientific researches. Quinidine is the dextroisomer of quinine and has the similar pharmacological properties as quinine, but quinidine’s effects are five to ten times stronger on the heart than quinine.
The Uses of Quinidine
Quinidine occurs in cinchona bark to about0.25–0.3% and also in cuprea bark. It is present in quinine sulfate mother liquor. Itis formed by isomerization of quinine. Itis used in the prevention of certain cardiacarrhythmias.
The Uses of Quinidine
A dextrorotatory stereoisomer of Quinine. Antiarrhythmic (class IA). Antimalarial
The Uses of Quinidine
antiarrhythmic, antimalarial
Background
Quinidine is a D-isomer of quinine present in the bark of the Cinchona tree and similar plant species. This alkaloid was first described in 1848 and has a long history as an antiarrhythmic medication. Quinidine is considered the first antiarrhythmic drug (class Ia) and is moderately efficacious in the acute conversion of atrial fibrillation to normal sinus rhythm. It prolongs cellular action potential by blocking sodium and potassium currents. A phenomenon known as “quinidine syncope” was first described in the 1950s, characterized by syncopal attacks and ventricular fibrillation in patients treated with this drug. Due to its side effects and increased risk of mortality, the use of quinidine was reduced over the next few decades. However, it continues to be used in the treatment of Brugada syndrome, short QT syndrome and idiopathic ventricular fibrillation.
Indications
Quinidine is indicated for the management and prophylactic therapy of atrial fibrillation/flutter, as well as the suppression of recurrent documented ventricular arrhythmias. It is also used in the treatment of Brugada syndrome, short QT syndrome and idiopathic ventricular fibrillation..
What are the applications of Application
Quinidine is a dextrorotatory stereoisomer of Quinine
Definition
ChEBI: A cinchona alkaloid consisting of cinchonine with the hydrogen at the 6-position of the quinoline ring substituted by methoxy.
Indications
Quinidine acts as a class I antiarrhythmic agent (Ia) in the heart. It was clinically applicable to the treatment of recurrent, documented, life-threatening ventricular arrhythmias .
brand name
Duraquin (Warner Chilcott); Quinaglute (Berlex).
Biological Functions
Quinidine is an alkaloid obtained from various species of Cinchona or its hybrids, from Remijia pedunculata, or from quinine. Quinidine is the dextrorotatory isomer of quinine.Quinidine (Quinidex) was one of the first clinically used antiarrhythmic agents. Because of the high incidence of ventricular proarrhythmia associated with its use and numerous other equally efficacious agents, quinidine is now used sparingly. Quinidine shares all of the pharmacological properties of quinine, including antimalarial, antipyretic, oxytocic, and skeletal muscle relaxant actions.
General Description
Crystals or white powder.
Air & Water Reactions
Insoluble in water.
Hazard
Poison.
Health Hazard
Quinidine is more potent than quinine in itsaction on the cardiovascular system. Overdosesmay cause lowering of blood pressure.Gastric effects are lower than quinine. Toxicityis lower relative to quinine; subcutaneouslethal dose in mice is 400 mg/kg against200 mg/kg for quinine.
Fire Hazard
Flash point data for Quinidine are not available. Quinidine is probably combustible.
Biochem/physiol Actions
Class IA antiarrhythmic; potassium channel blocker.
Mechanism of action
Quinidine generally is administered orally, although slow intravenous administration also is possible . Quinidine is the prototype of class I antiarrhythmics; therefore, substances in this group also are called “quinidine-like.”However, it differs, for example, from lidocaine by the following effects: it increases the duration of the action potential, in the ECG it increases QRS duration and Q– T interval, and the refractory period is markedly prolonged. Quinidine can accelerate the impulse conduction rate in the AV node (atropine-like effect).
Pharmacokinetics
Quinidine is an antimalarial schizonticide, and a class Ia antiarrhythmic agent used to interrupt or prevent reentrant arrhythmias and arrhythmias due to increased automaticity, such as atrial flutter, atrial fibrillation, and paroxysmal supraventricular tachycardia. In most patients, quinidine can lead to an increase in the sinus rate. Quinidine also causes a marked prolongation of the QT interval in a dose-related manner, acts peripherally as an α-adrenergic antagonist, and has anticholinergic and negative inotropic activity.
The QT interval prolongation caused by quinidine can lead to increased ventricular automaticity and polymorphic ventricular tachycardias, such as torsades de pointes. The risk of torsades is increased by bradycardia, hypokalemia, hypomagnesemia or high serum levels of quinidine. However, this type of rhythm disturbance may appear in the absence of any of them. Patients treated with quinidine may also be at risk of a paradoxical increase in ventricular rate in atrial flutter/fibrillation, and patients with sick sinus syndrome treated with quinidine may develop marked sinus node depression and bradycardia.
Pharmacology
Quinidine exhibits all of the pharmacological properties of quinine, including antimalarial, fever-reducing, and other properties. Quinidine is used in various forms of arrhythmia for preventing tachycardia and atrial fibrillation, and particularly for preventing ciliary fibrillation, paroxysmal supraventricular tachycardia, extrasystole, and ventricular tachycardia. However, it is a toxic drug and is used relatively rarely. It is also prescribed under the name cardioquin, duraquin, quinidex, and others.
Clinical Use
Primary indications for the use of quinidine include (1) abolition of premature complexes that have an atrial, A-V junctional, or ventricular origin; (2) restoration of normal sinus rhythm in atrial flutter and atrial fibrillation after controlling the ventricular rate with digitalis; (3) maintenance of normal sinus rhythm after electrical conversion of atrial arrhythmias; (4) prophylaxis against arrhythmias associated with electrical countershock; (5) termination of ventricular tachycardia; and (6) suppression of repetitive tachycardia associated with Wolff- Parkinson-White (WPW) syndrome.
Although quinidine often is successful in producing normal sinus rhythm, its administration in the presence of a rapid atrial rate (flutter and possibly atrial fibrillation) can lead to a further and dangerous increase in the ventricular rate secondary to inhibition of basal vagal tone upon the A-V node. For this reason, digitalis should be used before quinidine when one is attempting to convert atrial flutter or atrial fibrillation to normal sinus rhythm.
Side Effects
The most common adverse effects associated with
quinidine administration are diarrhea (35%), upper
gastrointestinal distress (25%), and light-headedness (15%). Other relatively common adverse effects include
fatigue, palpitations, headache (each occurring
with an incidence of 7%), anginalike pain, and rash.
These adverse effects are generally dose related and reversible
with cessation of therapy. In some patients,
quinidine administration may bring on thrombocytopenia
due to the formation of a plasma protein–quinidine
complex that evokes a circulating antibody directed
against the blood platelet. Although platelet counts return
to normal on cessation of therapy, administration
of quinidine or quinine at a later date can cause the
reappearance of thrombocytopenia.
The cardiac toxicity of quinidine includes A-V and intraventricular block, ventricular tachyarrhythmias, and depression of myocardial contractility. Ventricular arrhythmia induced by quinidine leading to a loss of consciousness has been referred to as quinidine syncope. This devastating side effect is more common in women than in men and may occur at therapeutic or subtherapeutic plasma concentrations. Large doses of quinidine can produce a syndrome known as cinchonism, which is characterized by ringing in the ears, headache, nausea, visual disturbances or blurred vision, disturbed auditory acuity, and vertigo. Larger doses can produce confusion, delirium, hallucinations, or psychoses.Quinidine can decrease blood glucose concentrations, possibly by inducing insulin secretion.
Safety Profile
Poison by ingestion, subcutaneous, intravenous, intramuscular, and intraperitoneal routes. A skin irritant. Implicated in aplastic anemia. When heated to decomposition it emits toxic fumes of NOx.
Drug interactions
Quinidine can increase the plasma concentrations of
digoxin, which may in turn lead to signs and symptoms of
digitalis toxicity. Gastrointestinal, central nervous system
(CNS), or cardiac toxicity associated with elevated
digoxin concentrations may occur.Quinidine and digoxin
can be administered concurrently; however, a downward
adjustment in the digoxin dose may be required.
Drugs that have been associated with elevations in
quinidine concentrations include acetazolamide, the
antacids magnesium hydroxide and calcium carbonate,
and the H2-receptor antagonist cimetidine. Cimetidine
inhibits the hepatic metabolism of quinidine. Phenytoin,
rifampin, and barbiturates increase the hepatic metabolism
of quinidine and reduce its plasma concentrations.
Metabolism
Quinidine is mainly metabolized in the liver by cytochrome P450 enzymes, specifically CYP3A4. The major metabolite of quinidine is 3-hydroxy-quinidine, which has a volume of distribution larger than quinidine and an elimination half-life of about 12 hours. Non-clinical and clinical studies suggest that 3-hydroxy-quinidine has approximately half the antiarrhythmic activity of quinidine; therefore, this metabolite is partly responsible for the effects detected with the chronic use of quinidine.
Metabolism
Quinidine's bioavailability appears to depend on a combination of metabolism and P-gp efflux. The bioavailabilities of quinidine sulfate and gluconate are 80 to 85% and 70 to 75%, respectively. Once absorbed, quinidine is subject to hepatic first-pass metabolism and is approximately 85% plasma protein bound, with an elimination half-life of approximately 6 hours. Quinidine is metabolized mainly in the liver, and renal excretion of unchanged drug also is significant (~10–50%). The metabolites are hydroxylated derivatives at either the quinoline ring through first-pass O-demethylation or at the quinuclidine ring through oxidation of the vinyl group. These metabolites possess only about one-third the activity of quinidine. Their contribution to overall therapeutic effect of quinidine is unclear. Recently, the clinical significance of the well-documented digoxin–quinidine interaction was described previously under digoxin–drug interactions. Apparently, quinidine (a P-gp substrate) inhibits the renal tubular secretion of digoxin via the P-gp efflux pump, resulting in increased plasma concentration for digoxin.
storage
Store at -20°C
Purification Methods
Crystallise it from *C6H6 or dry CHCl3/pet ether (b 40-60o), discarding the initial, oily crop of crystals. Dry it under vacuum at 100o over P2O5. It has been used as a chiral catalyst [Wynberg & Staring J Am Chem Soc 104 166 1982, J Org Chem 50 1977 1985]. [Beilstein 23 H 506, 23 I 164, 23 II 414, 23 III/IV 3261, 23/13 V 395.]
Precautions
One of the few absolute contraindications for quinidine
is complete A-V block with an A-V pacemaker or idioventricular
pacemaker; this may be suppressed by
quinidine, leading to cardiac arrest.
Persons with congenital QT prolongation may develop
torsades de pointes tachyarrhythmia and should
not be exposed to quinidine.
Owing to the negative inotropic action of quinidine,
it is contraindicated in congestive heart failure and hypotension.
Digitalis intoxication and hyperkalemia can accentuate
the depression of conduction caused by quinidine.
Myasthenia gravis can be aggravated severely by
quinidine’s actions at the neuromuscular junction.
The use of quinidine and quinine should be avoided
in patients who previously showed evidence of quinidine-
induced thrombocytopenia.
References
Wit, Andrew L. "The Effects of Quinidine on the Cellular Electrophysiology of the Heart: A Brief Review." Journal of Cardiovascular Electrophysiology 3.5(2010):316-322.
Alloway, J. A., and M. P. Salata. "Quinidine-induced rheumatic syndromes. " Seminars in Arthritis & Rheumatism 24.5(1995):315-322.
Nakano, J, and M. C. R. Jr. "Effect of quinidine on cardiovascular dynamics." Arch Int Pharmacodyn Ther 168.2(1967):400-416.
Properties of Quinidine
| Melting point: | 168-172 °C(lit.) |
| alpha | 256 º (c=1, EtOH) |
| Boiling point: | 462.75°C (rough estimate) |
| Density | 1.1294 (rough estimate) |
| refractive index | 1.5700 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | insoluble in H2O; ≥10.32 mg/mL in EtOH with ultrasonic; ≥11.95 mg/mL in DMSO |
| form | Powder |
| pka | 5.4, 10.0(at 20℃) |
| color | White to off-white |
| optical activity | [α]20/D +265±5°, c = 0.8% in ethanol (dry matter) |
| Water Solubility | 0.05 g/100 mL (20 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,8060 |
| BRN | 91866 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 56-54-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Quinine(56-54-2) |
| EPA Substance Registry System | Quinidine (56-54-2) |
Safety information for Quinidine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Quinidine
New Products
Tetrabutylammonium iodide (3,3-DIFLUOROCYCLOBUTYL)METHANOL 4,4-DIFLUOROCYCLOHEXANAMINE Cyclobutylamine (S)-3-Fluoro-pyrrolidine hydrochloride 3-Oxocyclobutanecarboxylic acid N-Hydroxy-2-methylpropanimidamide L-tert-Leucine,97% 2-Bromophenylacetonitrile, 97% Aluminum oxide, basic Calcium hydroxide, 95% Diallylamine, 99% 2-Iodobenzoic Acid 3-Methoxybenzonitrile Pentachlorobenzonitrile Chloral Dibenzoyl Peroxide Titanium Dioxide O-Benzylhydroxylamine Hydrochloride 2-Nitrobenzaldehyde 2-Picolylamine (2-Aminomethylpyridine) 2-Venylpyridine Ethyl-2-Chloroacetoacetate 4-Dimethylamine PyridineRelated products of tetrahydrofuran
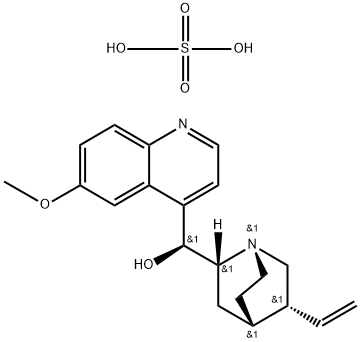
You may like
-
 Quinidine 98%View Details
Quinidine 98%View Details -
 56-54-2 99%View Details
56-54-2 99%View Details
56-54-2 -
 Quinidine CAS 56-54-2View Details
Quinidine CAS 56-54-2View Details
56-54-2 -
 Quinidine CAS 56-54-2View Details
Quinidine CAS 56-54-2View Details
56-54-2 -
 QUINIDINE CAS 56-54-2View Details
QUINIDINE CAS 56-54-2View Details
56-54-2 -
 Quinidine CAS 56-54-2View Details
Quinidine CAS 56-54-2View Details
56-54-2 -
 TETRABUTYLAMMONIUM CYANIDE 10442-39-4 98+View Details
TETRABUTYLAMMONIUM CYANIDE 10442-39-4 98+View Details
10442-39-4 -
 19752-84-2 TETRAHYDRO-2H-PYRAN-3-OL 98+View Details
19752-84-2 TETRAHYDRO-2H-PYRAN-3-OL 98+View Details
19752-84-2
