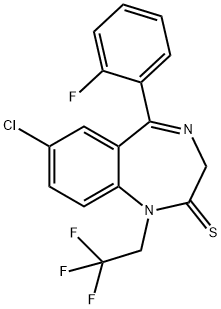
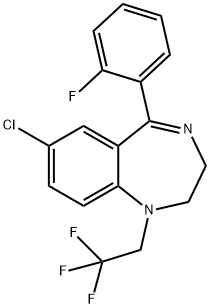
QUAZEPAM
Synonym(s):7-Chloro-5-(2-fluorophenyl)-1,3-dihydro-1-(2,2,2-trifluoroethyl)- 2H-1,4-benzodiazepine-2-thione
- CAS NO.:36735-22-5
- Empirical Formula: C17H11ClF4N2S
- Molecular Weight: 386.79
- MDL number: MFCD00868025
- EINECS: 253-179-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is QUAZEPAM ?
Absorption
The half-life of absorption following oral administration is approximately 30 minutes. Peak plasma concentration (Cmax) is approximately 20 ng/mL and occurs around 2 hours following administration.
Toxicity
Benzodiazepine overdose is typically characterized by central nervous system depression ranging from mild drowsiness to coma. Mild-to-moderate cases may involve relatively minor symptoms like drowsiness, dysarthria, confusion, and ataxia, while severe cases may lead to respiratory depression and coma. In rare cases, paradoxical disinhibitory reactions (e.g. agitation, violent behaviour, impulsivity) may occur.
The management of benzodiazepine overdose should involve general supportive measures as clinically indicated. Flumazenil is a benzodiazepine receptor antagonist which is indicated for the complete or partial reversal of benzodiazepine-induced sedation, although its appropriateness is highly dependent on the clinical status and history of the patient. The use of flumazenil can lead to withdrawal and significant adverse reactions (e.g. seizures), especially in the context of mixed overdoses and in long-term benzodiazepine users with an established physical dependency, and its use is contraindicated in patients using benzodiazepines for potentially life-threatening conditions (e.g. status epilepticus). If the decision is made to use flumazenil, it should be used as specified in its prescribing information and as an adjunct alongside general supportive management.
Description
Quazepam is a rapidly acting, short term hypnotic reported to exhibit a low incidence of side effects.
Originator
Schering (USA)
The Uses of QUAZEPAM
Quazepam is a benzodiazepine derivative drug and is known to bind nonspecifically to benzodiazepine receptors, which affects muscle relaxation, anticonvulsant activty, motor coordination and memory. Quazepam is known to induce motor impairment and exerts hypnotic properties. Quazepam is often used for the treatment of insomina.
Background
Quazepam is a trifluoroethyl benzodiazepine derivative. It was first approved in the US in 1985 and is used as a hypnotic for the treatment of insomnia.
It appears to be unique amongst other benzodiazepine derivatives in its relatively high affinity for sleep-promoting α1 subunit-containing GABAA receptors and low affinity for other receptors.
Indications
Quazepam is indicated for the treatment of insomnia characterized by difficulty falling asleep, frequent nocturnal awakenings, and/or early morning awakenings.
Definition
ChEBI: Quazepam is a benzodiazepine.
brand name
Doral (Questcor).
General Description
Quazepam (Doral) and its active metabolitesreportedly are relatively selective for the benzodiazepinemodulatory site on GABAA receptors with α1 subunitand are hypnotic agents. Quazepam is metabolized by oxidation to the 2-oxo compound and then N-dealkylation.Both metabolites are active; the first reportedly is the morepotent and selective. Thereafter, 3-hydroxylation and glucuronidationoccur.
Pharmacokinetics
Benzodiazepines, including quazepam, exert their sedative and anxiolytic effects by potentiating the effects of endogenous GABA, the primary inhibitory neurotransmitter in the central nervous system.
Metabolism
Quazepam undergoes extensive hepatic metabolism, primarily via CYP3A4 and to a lesser extent via CYP2C9, CYP2C19, and FMO1. Quazepam is first metabolized to 2-oxoquazepam, which is then further metabolized to both N-desalkyl-2-oxoquazepam (norflurazepam) and 3-hydroxy-2-oxoquazepam. Both 2-oxoquazepam and N-desalkyl-2-oxoquazepam exert CNS depressant activity.
Properties of QUAZEPAM
| Melting point: | 137.5-139° |
| Boiling point: | 429.9±55.0 °C(Predicted) |
| Density | 1.42±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| pka | 0.49±0.20(Predicted) |
| CAS DataBase Reference | 36735-22-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Quazepam(36735-22-5) |
Safety information for QUAZEPAM
Computed Descriptors for QUAZEPAM
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
