Protocatechualdehyde
Synonym(s):3,4-Dihydroxybenzaldehyde;Protocatechualdehyde
- CAS NO.:139-85-5
- Empirical Formula: C7H6O3
- Molecular Weight: 138.12
- MDL number: MFCD00003370
- EINECS: 205-377-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00
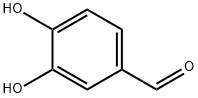
What is Protocatechualdehyde?
Description
3,4-Dihydroxybenzaldehyde has been recognized as one of the antifungal compound extracted from the outer skin of green Cavendish bananas. Protocatechualdehyde is extracted from roots of the common traditional Chinese medicine Salvia miltiorrhiza Bge, which was harvested in autumn for better quality. Now, the existence of protocatechualdehyde has been found in variety of herbs, for example, in the leaf of Stenoloma Chusanum (L.) Ching and Ilex chinensis Sims.
Chemical properties
Pale beige acicular crystal (in water or methylbenzene) or off-white powder, crystal twin shape. Solubility: easily soluble in ethanol, acetone, ethyl acetate, ethyl ether, and hot water; soluble in cold water; insoluble in benzene and chloroform. Specific optical rotation: easily oxidized to benzoquinone and changes color, and it is instable in water. protocatechualdehyde can be found in barley, grapevine, green Cavendish, and root of herb S. miltiorrhiza.
History
In the 1940s, the research was conducted overseas to obtain protocatechualdehyde form herbs. In 1972, the chemical group of Chinese herbal medicine in Nanjing College of Pharmacy systematically studied chemical composition in purple flower holly leaf and separated six monomers including protocatechualdehyde. Then they compared the effect of protocatechualdehyde, Salvia miltiorrhiza injection and hairy holly root injection, in which protocatechualdehyde is the most effective in increasing coronary sinus flow. Further experiments examined that protocatechualdehyde can increase coronary flow and improve coronary circulation, so it was called after perhexiline. Further clinical observation indicated that protocatechualdehyde has effect on coronary heart disease. The graduates of Nanjing College of Pharmacy in 1975 extracted, separated, isolated, and identified protocatechualdehyde in Salviamiltiorrhiza during the internship in Nanjing Municipal Hospital and studied its distribution, excretion, and toxicity in animals for clinical rational drug usage
The Uses of Protocatechualdehyde
3,4-Dihydroxybenzaldehyde is used in as an apoptosis inducer of human leukemia cells. It may be used for the surface modification of nanocrystalline TiO2 particles. Electrodeposited layer of 3,4-dihydroxybenzaldehyde may be used as effective redox mediator during oxidation of NADH at graphene. It may be used in the preparation of new diSchiff base ligands, which forms di-, tri- and tetranuclear Co(II) and Cu(II) complexes.
Definition
ChEBI: 3,4-dihydroxybenzaldehyde is a dihydroxybenzaldehyde.
Clinical Use
Perhexiline injection containing protocatechualdehyde 100? mg/2? mL by intravenous infusion or intramuscular injection was used to treat coronary heart disease,
chest tightness, angina, and myocardial infarction.
Injection treats ischemic stroke and improves both symptoms and signs of patients.
Treating chronic hepatitis and early cirrhosis: relieving the symptoms, promoting
the recovery of liver function, and hepatosplenomegaly.
It can significantly improve the blood rheology index for patients with acute exacerbation of chronic pulmonary heart disease.
Treatment of peptic ulcer: a certain effect.
Others: it has effect on many diseases like viral myocarditis, embolism of central
retinal artery, thromboangiitis obliterans, scleredema neonatorum, scleroderma,
psoriasis, nerve deafness, and toxemia of pregnancy.
Enzyme inhibitor
This aldehyde (FW = 138.12 g/mol), also known as 3,4- dihydroxybenzaldehyde and 4-formylcatechol, is soluble in water (5 g/100 mL at 20°C) and has a pKa value of 7.55 at 25°C. Protocatechualdehyde induces apoptosis in cytotoxic T cells. Target(s): aldehyde oxidase; aldose reductase; b-carotene 15,15’-monooxygenase; mandelonitrile lyase; protocatechuate 3,4-dioxygenase; protocatechuate 4,5- dioxygenase; tyrosinase, weakly inhibited, IC50 = 620 μM; and xanthine oxidase.
Purification Methods
Crystallise the aldehyde from water or toluene and dry it in a vacuum desiccator over KOH pellets or shredded wax respectively. [Beilstein 8 IV 1762.]
Properties of Protocatechualdehyde
| Melting point: | 150-157 °C(lit.) |
| Boiling point: | 213.5°C (rough estimate) |
| Density | 1.2667 (rough estimate) |
| refractive index | 1.4600 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | 6.3g/l |
| form | Crystalline Powder |
| pka | pK (25°) 7.55 |
| color | slightly brown |
| Odor | at 100.00 %. dry medicinal bitter almond |
| Water Solubility | 50 g/L (20 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,7893 |
| BRN | 774381 |
| Stability: | Stable. Incompatible with strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 139-85-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 3,4-Dihydroxybenzaldehyde(139-85-5) |
| EPA Substance Registry System | Benzaldehyde, 3,4-dihydroxy- (139-85-5) |
Safety information for Protocatechualdehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Protocatechualdehyde
Protocatechualdehyde manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
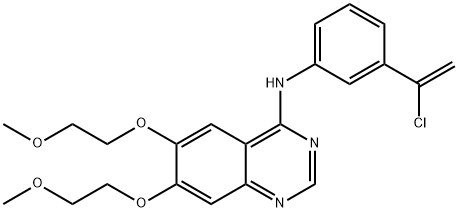


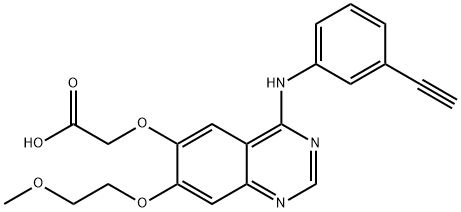
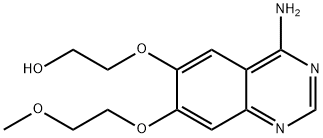
You may like
-
 139-85-5 3,4 -Dihydroxybenzaldehyde 98%View Details
139-85-5 3,4 -Dihydroxybenzaldehyde 98%View Details
139-85-5 -
 139-85-5 98%View Details
139-85-5 98%View Details
139-85-5 -
 3,4-Dihydroxy benzaldehyde 98%View Details
3,4-Dihydroxy benzaldehyde 98%View Details -
 139-85-5 1-methoxy-4-oxocyclohexane-1-carboxylic acid 98%+View Details
139-85-5 1-methoxy-4-oxocyclohexane-1-carboxylic acid 98%+View Details
139-85-5 -
 3,4-Dihydroxybenzaldehyde CAS 139-85-5View Details
3,4-Dihydroxybenzaldehyde CAS 139-85-5View Details
139-85-5 -
 3,4-Dihydroxybenzaldehyde CAS 139-85-5View Details
3,4-Dihydroxybenzaldehyde CAS 139-85-5View Details
139-85-5 -
 3,4-DihydroxybenzaldehydeView Details
3,4-DihydroxybenzaldehydeView Details
139-85-5 -
 3,4-Dihydroxybenzaldehyde Chemical, 50 Kg Bag, 99%View Details
3,4-Dihydroxybenzaldehyde Chemical, 50 Kg Bag, 99%View Details
139-85-5
