Propylphosphonic anhydride
Synonym(s):1-Propanephosphonic anhydride solution;2,4,6-Tripropyl-1,3,5,2,4,6-trioxatriphosphorinane-2,4,6-trioxide solution;PPACA;T3P
- CAS NO.:68957-94-8
- Empirical Formula: C9H21O6P3
- Molecular Weight: 318.18
- MDL number: MFCD00006583
- EINECS: 422-210-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:35
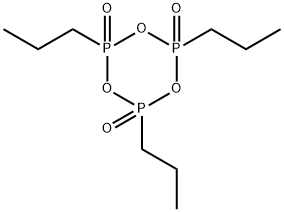
What is Propylphosphonic anhydride?
Description
T3P is typically sold as a 50% solution in a solvent (ex. EtOAc or DMF).
Chemical properties
Clear light yellow solution
The Uses of Propylphosphonic anhydride
Propylphosphonic anhydride may be used in the following studies:
- As coupling agent for the synthesis of bispyridine-based ligands, which are used as bridging linkers in multinuclear platinum anticancer drugs.
- Microwave-assissted Fischer indolization of arylhydrazines.
- As acid activating agent for the direct synthesis of acid azides from carboxylic acids.
- One-pot synthesis of coumarins.
- Microwave-mediated synthesis of carbocyclic and heterocyclic fused quinolones.
- One-pot synthesis of 1,2,4-oxadiazoles, 1,3,4-oxadiazoles, and 1,3,4-thiadiazoles from carboxylic acids.
- Activation of the carboxyl group for hydroxyamidation and peptide coupling and in the one-pot conversion of carboxylic acids into hydroxamic acids.
The Uses of Propylphosphonic anhydride

To a solution of the SM (2.92 g, 9.89 mmol) in 2-methyl tetrahydrofuran (115 mL) was added DIEA (13.8 mL, 39.5 mmol) and cyclopropylamine (2.77 mL, 39.5 mmol). The resulting mixture was heated to 70 C and T3P (50% weight solution in DCE) (17.3 mL, 59.3 mmol) was added. The reaction mixture was heated at 70 C for 16 h and then diluted with EtOAc (200 mL). The mixture was then washed sequentially with sat aq Na2CO3 (100 mL), 10% aq citric acid (100 mL), and saturated aq Na2CO3 (100 mL). The combined org extracts were dried (MgSO4) and concentrated in vacuo to give an oil. The crude was purified by column chromatography (eluting with 50% EtOAc/heptane) to give a solid which was recrystallized with IPA to provide the product as a white solid. [2.46 g, 74%]
The Uses of Propylphosphonic anhydride
It is employed as an efficient promoter for the Lossen rearrangement to synthesis urea and carbamate derivatives.
General Description
Propylphosphonic anhydride (T3P) is a reactive n-propyl phosphonic acid cyclic anhydride. It is a mild and low toxic coupling agent used in peptide synthesis. T3P also acts as a promoter and water scavenger in the Friedl?nder annulation reaction. It participates in the conversion of carboxylic acids and amides into nitriles, formation of Weinreb amides, ester synthesis, dehydrations, oxidation of alcohols, isonitrile synthesis, synthesis of alkenes from alcohols and C-C coupling reactions. T3P delivers outstanding advantages over traditional reagents, such as broad functional group tolerance, low epimerization, and water-soluble by-products and hence gives high purity and yield of the product.
Flammability and Explosibility
Not classified
Properties of Propylphosphonic anhydride
| Boiling point: | 65 °C |
| Density | 1.069 g/mL at 25 °C |
| vapor pressure | 0Pa at 25℃ |
| refractive index | n |
| Flash point: | 25 °F |
| storage temp. | Flammables area |
| solubility | Miscible with dioxane, terahydrofuran, dimethyl formamide, polar and aprotic organic solvents. |
| form | Solution |
| color | Clear yellow to brownish |
| Water Solubility | 9.6g/L at 25℃ |
| Sensitive | Moisture Sensitive |
| BRN | 5079255 |
| Exposure limits | ACGIH: TWA 20 ppm (Skin) OSHA: TWA 40 ppm(70 mg/m3) NIOSH: IDLH 137 ppm(25 mg/m3); TWA 20 ppm(34 mg/m3) |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 68957-94-8(CAS DataBase Reference) |
Safety information for Propylphosphonic anhydride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H290:Corrosive to Metals H314:Skin corrosion/irritation H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P234:Keep only in original container. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Propylphosphonic anhydride
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 1-Propylphosphonic acid cyclic anhydride CAS 68957-94-8View Details
1-Propylphosphonic acid cyclic anhydride CAS 68957-94-8View Details
68957-94-8 -
 1-Propanephosphonic anhydride, 50 wt.% solution in Acetonitrile CAS 68957-94-8View Details
1-Propanephosphonic anhydride, 50 wt.% solution in Acetonitrile CAS 68957-94-8View Details
68957-94-8 -
 T3P 50% DMF solution CAS 68957-94-8View Details
T3P 50% DMF solution CAS 68957-94-8View Details
68957-94-8 -
 Propanephosphonic acid anhydride CASView Details
Propanephosphonic acid anhydride CASView Details -
 Propanephosphonic acid anhydride CASView Details
Propanephosphonic acid anhydride CASView Details -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4