Propionyl chloride
Synonym(s):Propionic acid chloride;Propionyl chloride
- CAS NO.:79-03-8
- Empirical Formula: C3H5ClO
- Molecular Weight: 92.52
- MDL number: MFCD00000745
- EINECS: 201-170-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
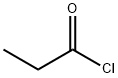
What is Propionyl chloride?
Chemical properties
Propionyl chloride is a colorless to light yellow, volatile, and readily flammable liquid, whose vapors hydrolyze in moist air. It is corrosive, lachrymatory, and has a strongly pungent odor. With water and lower alcohols it reacts vigorously under solvolysis to give the acid or ester. It is used for the introduction of the propionyl group and for the synthesis of propionate esters because of its high reactivity.
The Uses of Propionyl chloride
Propionyl chloride is used as an intermediate in the production of agrochemicals and pharmaceuticals. It serves as an intermediate for dyes, textile auxiliaries and peroxide compounds. It acts as a crop protecting agent.
What are the applications of Application
Propionyl chloride is an acid halide
Preparation
Propionyl chloride is synthesized by reacting propionic acid with phosphorus trichloride at 40-50°C for 1h, cooling, standing, separating and distilling to obtain the product.
CH3CH2COOH+PCl3→CH3CH2COCl+HOPCl2
Propionyl chloride is produced industrially by treatment of propionic acid with phosgene or thionyl chloride. The reaction occurs at ca. 50°C in the liquid phase in the presence of dialkylformamides. The product is then separated by distillation.
CH3CH2CO2H + COCl2 → CH3CH2COCl + HCl + CO2
Propionyl chloride can also be obtained by the reaction of propionic acid with PCl5.
What are the applications of Application
Propionyl chloride is used for the introduction of the propionyl group and for the synthesis of propionate esters because of its high reactivity. It is an chemical intermediate in the preparation of various propionic acid derivatives, It can also be used:
To convert anisole to 4-methoxypropiophenone and 2-methoxynaphthalene to 1-propio-2-methoxynaphthalene in the presence of Indium(III) chloride (InCl3) impregnated mesoporous Si-MCM-41 catalyst.
For chlorination in the presence of sulfuryl chloride and peroxides to form α-chloropropionyl chloride and β-chloropropionyl chloride.
In reaction with (hydroxypropyl)cellulose to form the propanoate ester, [(propionyloxy)propyl]cellulose.
General Description
Propionyl chloride appears as a colorless liquid with a pungent odor. Corrosive and very irritating to skin and eyes. Used to make other chemicals.
Air & Water Reactions
Highly flammable. Propionyl chloride reacts vigorously or violently with water to form propionic acid and hydrochloric acid [Merck 11th ed. 1989].
Reactivity Profile
Acid halides, such as Propionyl chloride, are water reactive; some are violently reactive. They are incompatible with strong oxidizing agents, alcohols, amines, and alkali. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Hazard
Strong irritant to skin.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Highly flammable
Safety Profile
A corrosive irritant to skin, eyes, and mucous membranes. Dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Reacts with water or steam to produce toxic and corrosive fumes. Exothermic reaction with diisopropyl ether produces much gas. The reaction may be dangerous if confmed. To fight fire, use CO2, dry chemical; do not use water. When heated to decomposition it emits lughly toxic fumes of Cl-. See also HYDROCHLORIC ACID.
Properties of Propionyl chloride
| Melting point: | -94 °C |
| Boiling point: | 77-79 °C (lit.) |
| Density | 1.059 g/mL at 25 °C (lit.) |
| vapor density | 3.2 (vs air) |
| vapor pressure | 106 hPa (20 °C) |
| refractive index | n |
| Flash point: | 53 °F |
| storage temp. | Store below +30°C. |
| form | Liquid |
| color | Clear |
| PH Range | <7.0 |
| explosive limit | 3.6-11.9%(V) |
| Water Solubility | REACTS |
| FreezingPoint | -94℃ |
| Sensitive | Moisture Sensitive |
| Merck | 14,7828 |
| BRN | 385632 |
| Exposure limits | ACGIH: TWA 0.1 ppm OSHA: TWA 0.1 ppm(0.4 mg/m3) NIOSH: IDLH 2 ppm; TWA 0.1 ppm(0.4 mg/m3); Ceiling 0.2 ppm(0.8 mg/m3) |
| CAS DataBase Reference | 79-03-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanoyl chloride(79-03-8) |
| EPA Substance Registry System | Propanoyl chloride (79-03-8) |
Safety information for Propionyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H302:Acute toxicity,oral H314:Skin corrosion/irritation H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Propionyl chloride
| InChIKey | RZWZRACFZGVKFM-UHFFFAOYSA-N |
Propionyl chloride manufacturer
JSK Chemicals
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran

You may like
-
 Propionyl Chloride extrapure AR (Specially purified) CAS 79-03-8View Details
Propionyl Chloride extrapure AR (Specially purified) CAS 79-03-8View Details
79-03-8 -
 Propionyl chloride CAS 79-03-8View Details
Propionyl chloride CAS 79-03-8View Details
79-03-8 -
 Propionyl chloride 95% CAS 79-03-8View Details
Propionyl chloride 95% CAS 79-03-8View Details
79-03-8 -
 Propionyl chloride CAS 79-03-8View Details
Propionyl chloride CAS 79-03-8View Details
79-03-8 -
 Propionyl Chloride CAS 79-03-8View Details
Propionyl Chloride CAS 79-03-8View Details
79-03-8 -
 Propionyl Chloride CASView Details
Propionyl Chloride CASView Details -
 Propionyl chloride CAS 79-03-8View Details
Propionyl chloride CAS 79-03-8View Details
79-03-8 -
 Propionyl chloride CAS 79-03-8View Details
Propionyl chloride CAS 79-03-8View Details
79-03-8
