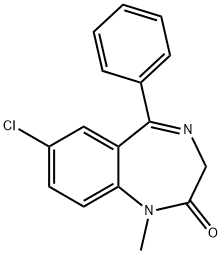
PRAZEPAM
- CAS NO.:2955-38-6
- Empirical Formula: C19H17ClN2O
- Molecular Weight: 324.8
- MDL number: MFCD00057905
- EINECS: 220-975-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
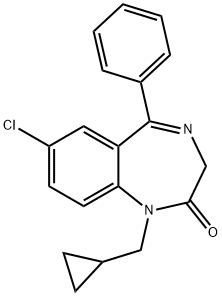
What is PRAZEPAM?
Toxicity
Symptoms of overdose include somnolence, confusion, coma, and diminished reflexes. Respiration, pulse and blood pressure should be monitored.
Description
Prazepam (Item No. 18358) is an analytical reference material categorized as a benzodiazepine. Prazepam is regulated as a Schedule IV compound in the United States. This product is intended for research and forensic applications.
Description
Prazepam (CRM) (Item No. 21701) is a certified reference material categorized as a benzodiazepine. Prazepam is regulated as a Schedule IV compound in the United States. Prazepam (CRM) (Item No. 21701) is provided as a DEA exempt preparation. This product is intended for research and forensic applications.
Originator
Demetrin, Goedecke, W. Germany,1973
The Uses of PRAZEPAM
Sedative-hypnotic.
Indications
For the treatment of anxiety disorders.
Background
Prazepam is a benzodiazepine that is used in the treatment of anxiety disorders. It is a schedule IV drug in the U.S.
Definition
ChEBI: Prazepam is a benzodiazepine.
Manufacturing Process
Preparation of 2-Cyclopropylcarbonylamido-5-Chlorobenzophenone: To 400.5 g (1.73 mols) of 2-amino-5-chlorobenzophenone dissolved in 220 g (2.18 mols) of triethylamine and 3.5 liters of tetrahydrofuran is added cautiously 181 g (1.73 mols) of cyclopropanecarboxylic acid chloride. The reaction is refluxed 2? hours and allowed to cool to room temperature. The solvent is then removed under vacuum to obtain 2-cyclopropylcarbonylamido-5chlorobenzophenone as a residue which is dissolved in 1 liter of methylene chloride, washed twice with 5% hydrochloric acid, and then twice with 10% potassium hydroxide. The methylene chloride solution is then dried over anhydrous magnesium sulfate, filtered and the solvent removed under vacuum. The residue is recrystallized from 1,500 ml of methanol, charcoaltreating the hot solution to give 356 g of 2-cyclopropylcarbonylamido-5chlorobenzophenone, MP 105° to 105.5°C (69% yield).
Preparation of 2-Cyclopropylmethylamino-5-Chlorobenzhydrol: To a slurry of 94.8 g (2.47 mols) of lithium aluminum hydride in 1.2 liters of tetrahydrofuran is added with stirring a solution of 356 g (1.18 mols) of 2cyclopropylcarbonylamido-5-chlorobenzophenone in 1.8 liters of tetrahydrofuran. The addition takes 80 minutes while maintaining gentle refluxing, and the reaction mixture is then refluxed overnight and allowed to cool to room temperature over a period of 3 days. The complex formed in the reaction mixture is then hydrolyzed with water.
During the hydrolysis, 500 ml of tetrahydrofuran is added to facilitate stirring. At a point where the flocculant white precipitate settles quickly when stirring is interrupted, the mixture is filtered, the filter cake washed with solvent, the combined filtrates dried over magnesium sulfate, filtered and the solvent removed under vacuum to obtain 2-cyclopropylmethylamino-5chlorobenzhydrol as a residue. The residue is recrystallized from 1,300 ml of Skelly B, giving 315 g of 2-cyclopropylmethylamino-5-chlorobenzhydrol, MP 85° to 85.5°C (93% yield).
Preparation of 2-Cyclopropylmethylamino-5-Chlorobenzophenone: To a solution of 315 g (1.09 mols) of 2-cyclopropylmethylamino-5-chlorobenzhydrol in 4 liters of benzene is added 453.6 g (5.22 mols) of manganese dioxide, freshly prepared according to the method of Attenburrow et al, J.C.S. 1952, 1104. The mixture is then refluxed for 1? hours, filtered, and the filtrate evaporated under vacuum. The reddish residue is recrystallized from 510 ml of 90% acetone-10% water, giving 181 g of pure 2-cyclopropylmethylamino5-chlorobenzophenone, MP 79° to 80°C (58% yield). Upon concentration of the mother liquor a second crop of 2-cyclopropylmethylamino-5chlorobenzophenone weighing 34.1 g and melting at 76.5°-78°C are obtained.
Preparation of 2-(N-Phthalimidoacetyl-N-Cyclopropylmethyl) -Amino-5Chlorobenzophenone: To a solution of 36.0 g (0.126 mol) of 2cyclopropylmethylamino-5-chlorobenzophenone in 500 ml of tetrahydrofuran is added 50.7 g (0.252 mol) of phthalimidoacetyl chloride. The resulting solution is refluxed for 16 to 24 hours, the solvent removed under vacuum, the residual oil crystallized from 200 ml of ethanol and recrystallized from 500 ml of 80% ethanol-20% tetrahydrofuran giving 44.7 g of 2-(N-phthalimidoacetylN-cyclopropylmethyl)-amino-5-chlorobenzophenone, MP 163° to 164°C (75% yield).
Preparation of 1-Cyclopropylmethyl-5-Phenyl-7-Chloro-1H-1,4-Benzodiazepine2(3H)-one: To a solution of 39.5 g (0.0845 mol) of 2-(N-phthalimidoacetyl-Ncyclopropylmethyl)amino5-chlorobenzophenone in a mixture of 423 ml of chloroform and 423 ml of ethanol is added 9.52 g (0.1903 mol) of hydrazine hydrate and 9.52 ml of water. This solution is allowed to stand at room temperature. In 3 hours a precipitate begins to form in the solution. After standing 16 to 24 hours a voluminous pulpy white precipitate forms. The solvents are removed under vacuum while keeping the temperature under 40°C and the residue is partitioned between dilute ammonia water and ether.
The aqueous layer is separated and washed with ether, the ether extracted with 5% hydrochloric acid, the acidic solution is made basic with 10% sodium hydroxide and again extracted with ether. Since some spontaneous crystallization occurs in the ether, the solvent is removed without drying under vacuum and the residue is recrystallized from 35 ml of ethanol giving 18.0 g of 1-cyclopropylmethyl-5-phenyl-7-chloro-1H-1,4-benzodiazepine-2(3H)-one, MP 145° to 146°C (65% yield), according to US Patent 3,192,199.
brand name
Centrax (Parke-Davis).
Therapeutic Function
Tranquilizer
General Description
Prazepam, 7-chloro-1-(cyclopropylmethyl)-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepine-2-one(Verstran), has a long overall half-life. Extensive N-dealkylationoccurs to yield active nordazepam. 3-Hydroxylation ofboth prazepam and nordazepam occurs.
Pharmacokinetics
Prazepam is a benzodiazepine derivative with anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant activity. Benzodiazepines may be habit-forming (causing mental or physical dependence), especially when taken for a long time or in high doses.
Metabolism
Hepatic.
Properties of PRAZEPAM
| Melting point: | 145-146° |
| Boiling point: | 538.5±50.0 °C(Predicted) |
| Density | 1.1660 (rough estimate) |
| refractive index | 1.5790 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | Dissolve 0.25 g in alcohol R and dilute to 10 mL with the same solvent. The solution is clear (2.2.1) and colourless (2.2.2, Method II). |
| pka | pKa 2.94 (Uncertain) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 9.095mg/L(25 ºC) |
| IARC | 3 (Vol. 66) 1996 |
| EPA Substance Registry System | 2H-1,4-Benzodiazepin-2-one, 7-chloro-1-(cyclopropylmethyl)-1,3-dihydro-5-phenyl- (2955-38-6) |
Safety information for PRAZEPAM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for PRAZEPAM
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran
You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5