PRASEODYMIUM
Synonym(s):;Praseodymium
- CAS NO.:7440-10-0
- Empirical Formula: Pr
- Molecular Weight: 140.91
- MDL number: MFCD00011174
- EINECS: 231-120-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
What is PRASEODYMIUM?
Chemical properties
grey powder
Physical properties
Praseodymium is a silvery-white, soft metal that is easily formed into various shapes. Whenthe pure metal is exposed to the air, a green oxide coating forms on its surface. To preventoxidation, praseodymium is usually kept in oil in a covered container.
Its melting point is 931°C, its boiling point is 3,520°C, and its density is 6.77g/cm3.
Isotopes
There are 45 isotopes of praseodymium. All are artificially produced and radioactivewith half-lives ranging from several hundred nanoseconds to 23.6 days. Only oneis stable (Pa-141), and it makes up 100% of the praseodymium found in the Earth’scrust.
Origin of Name
The name is derived from two Greek words, prasios and didymos, which together mean “green twins.”
Occurrence
Praseodymium is the 41st most abundant element on Earth and is found in the ores of monazite,cerite, bastnasite, and allanite along with other rare-earths. Praseodymium is also the stableisotope resulting from the process of fission of some other heavy elements, such as uranium.
Praseodymium is mainly found in monazite sands and bastnasite ores. The monazite sandscontain all of the rare-earths and are found in river sand in India and Brazil as well as inFlorida beach sand. A large deposit of bastnasite exists in California.
Praseodymium is separated from its ore and other rare-earths by a process called ionexchange, which exchanges one type of ion for another.
History
In 1879, Lecoq de Boisbaudran isolated a new earth, samaria, from didymia obtained from the mineral samarskite. Six years later, in 1885, von Welsbach separated didymia into two others, praseodymia and neodymia, which gave salts of different colors. As with other rare earths, compounds of these elements in solution have distinctive sharp spectral absorption bands or lines, some of which are only a few Angstroms wide. Praseodymium occurs along with other rare-earth elements in a variety of minerals. Monazite and bastnasite are the two principal commercial sources of the rare-earth metals. Ion-exchange and solvent extraction techniques have led to much easier isolation of the rare earths and the cost has dropped greatly. Thirty-seven isotopes and isomers are now recognized. Praseodymium can be prepared by several methods, such as by calcium reduction of the anhydrous chloride or fluoride. Misch metal, used in making cigarette lighters, contains about 5% praseodymium metal. Praseodymium is soft, silvery, malleable, and ductile. It was prepared in relatively pure form in 1931. It is somewhat more resistant to corrosion in air than europium, lanthanum, cerium, or neodymium, but it does develop a green oxide coating that splits off when exposed to air. As with other rare-earth metals it should be kept under a light mineral oil or sealed in plastic. The rare-earth oxides, including Pr2O3, are among the most refractory substances known. Along with other rare earths, it is widely used as a core material for carbon arcs used by the motion picture industry for studio lighting and projection. Salts of praseodymium are used to color glasses and enamels; when mixed with certain other materials, praseodymium produces an intense and unusually clean yellow color in glass. Didymium glass, of which praseodymium is a component, is a colorant for welder’s goggles. The metal (99.9% pure) is priced at about $4/g.
Characteristics
As a metal, Pr is hygroscopic (adsorbs water) and tarnishes in the atmosphere. It will reactwith water to liberate hydrogen. It is soluble in acids and forms greenish salts, along with someother rare-earths. It is used to fabricate the electrodes for high-intensity lights.
The Uses of PRASEODYMIUM
Praseodymium salts, ingredient of mischmetal, core material for carbon arcs, colorant in glazes and glasses, catalyst, phosphors, lasers.
The Uses of PRASEODYMIUM
Praseodymium’s major use is as an alloying agent along with magnesium to produce highstrengthsteel that is used in airplane engines and automobiles parts. Notwithstanding its greenish color, another important use of praseodymium is as a yellow pigment to color glassand ceramics. Along with several other rare-earths, it is also used to form the electrodes forhigh-intensity arc lamps.
It is used to manufacture safety goggles that filter out strong yellow light (used in welding,for example). Misch metal uses about 5% Pr in the manufacture of cigarette lighter flints.
The Uses of PRASEODYMIUM
Praseodymium optical properties for use in amplification of telecommunication systems, including as a doping agent in fluoride fibers. It is also used in the scintillator for medical CAT scans. Used as additive for special steel, non-ferric alloy, hydrogen storage alloy, also reducer for making other rare Earth metal.
Definition
Pr. Metallic element of atomic number 59, group IIIB of the periodic table, one of the rare earth elements of the lanthanide group, aw 140.9077, valences = 3, 4. No stable isotopes.
Definition
A soft ductile malleable silvery element of the lanthanoid series of metals. It occurs in association with other lanthanoids. Praseodymium is used in several alloys, as a catalyst, and in enamel and yellow glass for eye protection. Symbol: Pr; m.p. 931°C; b.p. 3512°C; r.d. 6.773 (20°C); p.n. 59; r.a.m. 140.91.
Definition
praseodymium: Symbol Pr. A softsilvery metallic element belonging tothe lanthanoids; a.n. 59; r.a.m.140.91; r.d. 6.773; m.p. 931°C; b.p.3512°C. It occurs in bastnasite andmonazite, from which it is recoveredby an ion-exchange process. The onlynaturally occurring isotope ispraseodymium–141, which is notradioactive; however, fourteen radioisotopeshave been produced. It isused in mischmetal, a rare-earthalloy containing 5% praseodymium,for use in lighter ?ints. Another rareearthmixture containing 30%praseodymium is used as a catalyst in cracking crude oil. The element wasdiscovered by C. A. von Welsbach in1885.
Preparation
Praesodymium may be recovered from its minerals monazite and bastanasite. The didymia extract of rare earth minerals is a mixture of praesodymia and neodymia, primarily oxides of praesodymium and neodymium. Several methods are known for isolation of rare earths. These are applicable to all rare earths including praesodymium. They include solvent extractions,ionexchange, and fractional crystallization. While the first two methods form easy and rapid separation of rare earth metals, fractional crystallization is more tedious. Extractions and separations of rare earths have been discussed in detail earlier (see Neodymium and Cerium).
Praesodymium metal can be obtained from its anhydrous halides by reduction with calcium. The metal also may be prepared by electrolysis of fused praesodymium chloride at elevated temperatures (about 1,000°C).Alternatively, an eutectic mixture of praesodymium chloride, potassium chloride, and sodium chloride may be electrolyzed. In such electrolysis graphite is the anode and tungsten the cathode.
Hazard
If praseodymium gets wet or is submerged in water, the hydrogen released may explode. Itmust be kept dry and protected from the atmosphere.
Properties of PRASEODYMIUM
| Melting point: | 931 °C (lit.) |
| Boiling point: | 3520 °C (lit.) |
| Density | 6.71 g/mL at 25 °C (lit.) |
| storage temp. | Flammables area |
| form | powder |
| color | White |
| Specific Gravity | 6.782 |
| Resistivity | 68 μΩ-cm, 20°C |
| Water Solubility | Reacts with water. |
| Sensitive | Air & Moisture Sensitive |
| Merck | 13,7797 |
| Exposure limits | ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| CAS DataBase Reference | 7440-10-0(CAS DataBase Reference) |
| EPA Substance Registry System | Praseodymium (7440-10-0) |
Safety information for PRASEODYMIUM
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H250:Pyrophoric liquids; Pyrorophoric solids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P231+P232:Handle under inert gas. Protect from moisture. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. |
Computed Descriptors for PRASEODYMIUM
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
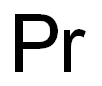


You may like
-
 Praseodymium foil, 0.25mm (0.01 in.) thick CAS 7440-10-0View Details
Praseodymium foil, 0.25mm (0.01 in.) thick CAS 7440-10-0View Details
7440-10-0 -
 Praseodymium foil, 1.0mm (0.04 in.) thick CAS 7440-10-0View Details
Praseodymium foil, 1.0mm (0.04 in.) thick CAS 7440-10-0View Details
7440-10-0 -
 Praseodymium CAS 7440-10-0View Details
Praseodymium CAS 7440-10-0View Details
7440-10-0 -
 Praseodymium CAS 7440-10-0View Details
Praseodymium CAS 7440-10-0View Details
7440-10-0 -
 Praseodymium CAS 7440-10-0View Details
Praseodymium CAS 7440-10-0View Details
7440-10-0 -
 Praseodymium CAS 7440-10-0View Details
Praseodymium CAS 7440-10-0View Details
7440-10-0 -
 Praseodymium rod, 12.7mm (0.5 in.) dia. CAS 7440-10-0View Details
Praseodymium rod, 12.7mm (0.5 in.) dia. CAS 7440-10-0View Details
7440-10-0 -
 Praseodymium rod, 12.7mm (0.5 in.) dia. CAS 7440-10-0View Details
Praseodymium rod, 12.7mm (0.5 in.) dia. CAS 7440-10-0View Details
7440-10-0