POTASSIUM HYDROSULFIDE
- CAS NO.:1310-61-8
- Empirical Formula: HKS
- Molecular Weight: 72.17
- MDL number: MFCD00061365
- EINECS: 215-182-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
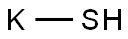
What is POTASSIUM HYDROSULFIDE?
Chemical properties
White to yellow crystals; hydrogen sul- fide odor. Forms the polysulfide when exposed to air. Hygroscopic; soluble in alco- hol and water.
The Uses of POTASSIUM HYDROSULFIDE
Separation of heavy metals.
General Description
POTASSIUM HYDROSULFIDE contains potassium hydrosulfide in solution. POTASSIUM HYDROSULFIDE will have a rotten egg like odor. POTASSIUM HYDROSULFIDE has a pH between 8.5-11.5. POTASSIUM HYDROSULFIDE is a transparent to dark green liquid. Contact with heat or acidic materials will evolve highly toxic hydrogen sulfide. The liquid is highly corrosive to the skin and eyes. Ingestion causes severe burning and corrosion in all portions of the gastrointestinal tract.
Air & Water Reactions
Addition of water to hydrosulfide may drop the pH and allow copious quantities of hydrogen sulfide to be produced. Acid accelerates the H2S production.
Reactivity Profile
Inorganic sulfides are generally basic and therefore incompatible with acids. Many of these compounds are reducing agents and therefore react vigorously with oxidizing agents, including inorganic oxoacids, organic peroxides and epoxides. Simple salts of sulfides (such as sodium, potassium, and ammonium sulfide) react vigorously with acids to release hydrogen sulfide gas
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Properties of POTASSIUM HYDROSULFIDE
| Melting point: | 450-510° forming a dark red liquid |
| Density | 1.70 |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Powder |
| color | white to off-white |
| Water Solubility | very soluble H2O, alcohol [MER06] |
| Sensitive | air sensitive, hygroscopic |
| Stability: | Air Sensitive, Hygroscopic |
| CAS DataBase Reference | 1310-61-8 |
| EPA Substance Registry System | Potassium sulfide (K(SH)) (1310-61-8) |
Safety information for POTASSIUM HYDROSULFIDE
Computed Descriptors for POTASSIUM HYDROSULFIDE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![POTASSIUM THIOCYANATE, [14C]](https://img.chemicalbook.in/StructureFile/ChemBookStructure1/GIF/CB3162840.gif)
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
