Potassium cyanate
Synonym(s):Cyanic acid potassium salt;Potassium cyanate
- CAS NO.:590-28-3
- Empirical Formula: CKNO
- Molecular Weight: 81.12
- MDL number: MFCD00011411
- EINECS: 209-676-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:59
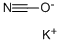
What is Potassium cyanate?
Chemical properties
Potassium cyanate, KOCN,is colorless,water soluble crystals. Used as an herbicide and for the manufacture of drugs and organic chemicals,
Chemical properties
Potassium cyanate, KCNO, white solid, soluble, formed along with lead metal by reaction of potassium cyanide and lead monoxide solids upon heating. Source of cyanate.
The Uses of Potassium cyanate
Potassium cyanate is used as chemical intermediate and as a weed killer. It is also used for the heat treatment of metals.
The Uses of Potassium cyanate
Herbicide, manufacture of organic chemicals and drugs, treatment of sickle-cell anemia.
The Uses of Potassium cyanate
Potassium cyanate is used as an inhibitor to prevent sickling of erythrocytes and has potential use as a treatment for sickle-cell anemia. Potassium cyanate is also used as a reagent to carbamylate proteins.
Definition
ChEBI: Potassium cyanate is a cyanate salt and a one-carbon compound. It has a role as a herbicide.
General Description
Potassium cyanate can be prepared by reacting urea with alkali hydroxide or carbonates at high temperatures.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Causes irritation of the gastrointestinal tract. An herbicide. It is said to be slowly metabolized in the body to cyanide but does not have high toxicity of cyanides. When heated to decomposition it emits very toxic fumes of CNand K2O.
Purification Methods
Common impurities include ammonia and bicarbonate ion (from hydrolysis). Purify it by preparing a saturated aqueous solution at 50o, neutralising with acetic acid, filtering, adding two volumes of EtOH and keeping for 3-4hours in an ice bath. (More EtOH can lead to co-precipitation of KHCO3.) Filter, wash it with EtOH and dry it rapidly in a vacuum desiccator (P2O5). The process is repeated [Vanderzee & Meyers J Chem Soc 65 153 1961].
Properties of Potassium cyanate
| Melting point: | 315 °C |
| Density | 2.056 g/mL at 25 °C (lit.) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Store below +30°C. |
| solubility | 750g/l |
| form | Crystalline Powder and Chunks |
| color | White |
| Specific Gravity | 2.056 |
| PH | 10 (50g/l, H2O, 20℃) |
| Water Solubility | 750 g/L (20 ºC) |
| Merck | 14,7625 |
| BRN | 3594798 |
| Boiling point: | 700-900°C (1000 hPa) Not applicable,(decomposition) |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Stability: | Stable. Incompatible with acids. |
| CAS DataBase Reference | 590-28-3(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium cyanate (590-28-3) |
Safety information for Potassium cyanate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium cyanate
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


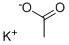
You may like
-
 Potassium cyanate CAS 590-28-3View Details
Potassium cyanate CAS 590-28-3View Details
590-28-3 -
 Potassium cyanate CAS 590-28-3View Details
Potassium cyanate CAS 590-28-3View Details
590-28-3 -
 Potassium cyanate CAS 590-28-3View Details
Potassium cyanate CAS 590-28-3View Details
590-28-3 -
 Potassium cyanate CAS 590-28-3View Details
Potassium cyanate CAS 590-28-3View Details
590-28-3 -
 Potassium cyanate CAS 590-28-3View Details
Potassium cyanate CAS 590-28-3View Details
590-28-3 -
 Potassium cyanate CAS 590-28-3View Details
Potassium cyanate CAS 590-28-3View Details
590-28-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
