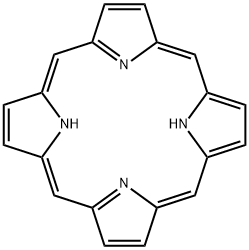
PORPHINE
- CAS NO.:101-60-0
- Empirical Formula: C20H14N4
- Molecular Weight: 310.35
- MDL number: MFCD00056811
- EINECS: 202-958-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is PORPHINE?
Description
Porphine (also called porphin) is a planar aromatic heterocyclic compound with a 12-carbon outside ring and four embedded pyrrole rings. It is a dark red crystalline solid that is soluble in some polar solvents such as pyridine and dioxane. It can be heated to 360 oC without melting.
Porphine was first prepared by German chemists Hans Fischer and Wilhelm Gleim in 1935. It is the parent structure of a large family of natural compounds called porphyrins, many of which are essential to life. Fischer was a 1930 Nobel Prize winner for his research on two porphyrins, heme (a constituent of hemoglobin) and chlorophyll. These porphyrins, like many others, contain a metal atom coordinated to the four nitrogen atoms.
Porphyria is a group of rare and often serious hereditary diseases in which the body is deficient in enzymes that modulate its proper distribution of porphyrins. Acute porphyrias cause significant damage to the nervous and digestive systems.
Less serious forms of the disease affect the skin, mostly in combination with sun exposure. Porphyrins are photosensitive, so exposure to intense light causes the skin to blister and burn—and sometimes turn red or purple.
So how does Halloween fit in with porphine, porphyrins, and porphyria? The combination of skin coloration and mental problems caused by porphyrias is, some have conjectured, the basis of vampire and werewolf folklore in central and eastern Europe. These theories have been largely debunked, but vampires and their kin live on, especially in TV series and Halloween costumes.
What are the applications of Application
Porphine is a parent compound for many porphyrins
Definition
ChEBI: Porphyrin is a member of porphyrins and a tetrapyrrole fundamental parent. It has a role as a metabolite.
Properties of PORPHINE
| Melting point: | 360°C |
| Boiling point: | 440.52°C (rough estimate) |
| Density | 1.3360 |
| refractive index | 1.6400 (estimate) |
| storage temp. | −20°C |
| EPA Substance Registry System | 21H,23H-Porphine (101-60-0) |
Safety information for PORPHINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for PORPHINE
PORPHINE manufacturer
Dr. Silviu Pharmachem Pvt., Ltd.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

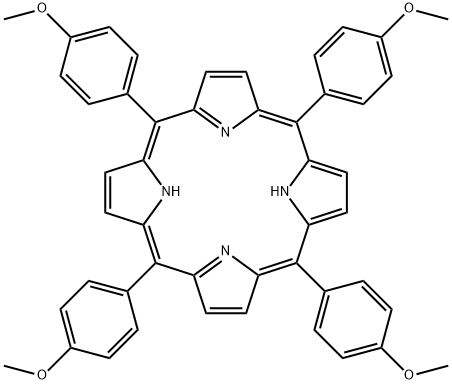
![5,10,15,20-TETRAKIS[4-(TRIMETHYLAMMONIO)PHENYL]-21H,23H-PORPHINE TETRA-P-TOSYLATE SALT](https://img.chemicalbook.in/CAS/GIF/69458-20-4.gif)
You may like
-
 101-60-0 / 681295-24-9 Porphine 98%View Details
101-60-0 / 681295-24-9 Porphine 98%View Details
101-60-0 / 681295-24-9 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1