Poly(vinyl alcohol)
Synonym(s):PVA;Poly(vinyl alcohol);AquaSolve;AtlasSupport;Parteck Polyvinyl alcohol
- CAS NO.:9002-89-5
- Empirical Formula: C2H4O
- Molecular Weight: 44.05256
- MDL number: MFCD00677796
- EINECS: 209-183-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02

What is Poly(vinyl alcohol)?
Absorption
Polyvinyl alcohol is poorly absorbed from gastrointestinal tract, and readily eliminated from the body .
Toxicity
The oral acute toxicity of PVOH (LD50) in rats and mice are 20 g/kg and 14.7 g/kg, respectively .
Adverse effects include eye pain and changes of vision, and redness or irritation of the eye .
Mild stinging or irritation of the eye, crusting of eyelid, eye pain, ocular hyperaemia, eye pruritus, foreign body sensation, eye discharge, and increased lacrimation may also occur .
Description
Poly(vinyl alcohol), made by removing acetate groups from poly(vinyl acetate), is a polymer used in a tremendous variety of products: as a vapor barrier in 2-L soda bottles, a thickening agent in shampoos, and the polymer that kids call "slime".
Chemical properties
Polyvinyl alcohol occurs as an odorless, white to cream-colored granular powder.
The Uses of Poly(vinyl alcohol)
NaA zeolite particles have been dispersed in a poly(vinyl alcohol) matrix to prepare a mixed-matrix membrane to study the pervaporative separation of water-butanol mixtures. Poly (vinyl alcohol)/gelatin based biocompatible polymeric scaffolds have been used to design for 3D cancer models.
The Uses of Poly(vinyl alcohol)
In the plastics industry in molding Compounds, surface coatings, films resistant to gasoline, textile sizes and finishing compositions; can be compounded to yield elastomers to be used in manufacture of artificial sponges, fuel hoses, etc., also in printing inks for plastics and glass, in pharmaceutical finishing, cosmetics, water-sol film and sheeting. Pharmaceutic aid (viscosity increasing agent); ophthalmic lubricant.
The Uses of Poly(vinyl alcohol)
Mowiol? 40-88 can be used for a variety of applications such as drug delivery, surface enhanced Raman spectroscopy (SERS) fiber-optic sensors, and transparent conducting oxides (TCOs).
Background
Polyvinyl alcohol is a water-soluble synthetic polymer obtained by polymerization of vinyl alcohol. It has varying roles in commercial and industrial applications such as papermaking, textiles, and printing. Polyvinyl alcohol is found in ophthalmic solutions as a lubricant to prevent irritation or to relieve dryness of the eyes .
Indications
For use as a lubricant to prevent further irritation or to relieve dryness of the eye(s) .
Definition
ChEBI: Polyvinyl alcohol is a homopolymer macromolecule obtained by polymerisation of vinyl alcohol. It is used as a pharmaceutic aid and ophthalmic lubricant as well as in the manufacture of surface coatings artificial sponges, cosmetics, and other products.
Production Methods
Polyvinyl alcohol is produced through the hydrolysis of polyvinyl acetate. The repeating unit of vinyl alcohol is not used as the starting material because it cannot be obtained in the quantities and purity required for polymerization purposes. The hydrolysis proceeds rapidly in methanol, ethanol, or a mixture of alcohol and methyl acetate, using alkalis or mineral acids as catalysts.
Preparation
Vinyl alcohol has not been isolated in the free state; the keto tautomer,
acetaldehyde, is much the more stable form and is always obtained:
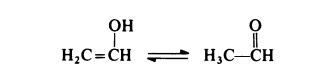
Thus poly(vinyl alcohol) cannot be prepared from its monomer by the usual techniques, although the polymerization of acetaldehyde with sodium amalgam at - 80 to - 20?? has been found to give poly( vinyl alcohol) of low molecular weight. For commercial purposes, poly(vinyl alcohol) is obtained exclusively from poly(vinyl acetate).
Poly(vinyl acetate) is readily hydrolysed by treating an alcoholic solution with aqueous acid or alkali. Acid hydrolysis results in traces of acid in the poly(vinyl alcohol) which are difficult to remove and which lead to instability of the polymer; alkaline hydrolysis results in contamination of the product by a large amount of sodium acetate which is also difficult to remove and which has little intrinsic value. These difficulties are avoided if poly(vinyl alcohol) is prepared from poly(vinyl acetate) by alcoholysis using a small amount of base as catalyst. The reaction is commonly carried out by treating poly(vinyl acetate) with methanol in the presence of sodium methoxide:
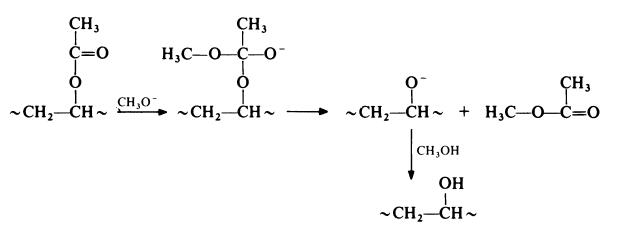
The preferred methods of preparing poly(vinyl acetate) for conversion to poly(vinyl alcohol) are solution and suspension polymerization. The former technique has the advantage that if polymerization is conducted in methanol the resulting solution can be used directly without the need for isolating the polymer; this method is the most suitable for continuous processes. Bulk polymerized poly(vinyl acetate) tends to give low molecular weight poly(vinyl alcohol) of poor colour.
In one continuous process, a solution of poly(vinyl acetate) in methanol (about 20%) is mixed with the catalyst solution in a high speed in-line mixer. The mixture then passes through a 'gelling zone' on a conveyor belt. Typically, the material is kept at 40?? for 10 minutes in this zone during which time the alcoholysis reaction occurs; poly(vinyl alcohol) is insoluble in methanol and a gel is produced. The gel is chopped up and neutralized with acetic acid to stop reaction; the liquid content (whica is mainly methanol and methyl acetate) is then expressed and recovered. The residual solid is washed with methanol, dried and pulverized.
It is possible to control the extent to which acetate groups are replaced by hydroxyl groups by changing the reaction conditions. In particular, the catalyst concentration and the time of reaction have a major effect on the degree of alcoholysis. The most common commercial types of poly(vinyl alcohol) are the so-called partially hydrolysed grades in which 87-89% of the acetate groups have been replaced and the completely hydrolysed grades in which 99-100% of the acetate groups have been replaced. The degree of alcoholysis has an effect on the properties of the polymer.
brand name
Liquifilm Tears (Allergan).
General Description
Polyvinyl alcohol (PVOH) is a hydrophilic linear polymer which forms copolymers of vinyl alcohol and vinyl acetate. Hence, the structural properties of polyvinyl alcohol polymers depend on the extent of polymerization and hydrolysis. Such changes cause both chemical and physical modifications such as esterification, etherification, crystallization, ion-polymer complexation in the polymer. Modified- PVOH structures are useful in biomedical applications.
Pharmaceutical Applications
Polyvinyl alcohol is used primarily in topical pharmaceutical and ophthalmic formulations. It is used as a stabilizing agent for emulsions (0.25–3.0% w/v). Polyvinyl alcohol is also used as a viscosity-increasing agent for viscous formulations such as ophthalmic products. It is used in artificial tears and contact lens solutions for lubrication purposes, in sustained-release formulations for oral administration, and in transdermal patches. Polyvinyl alcohol may be made into microspheres when mixed with a glutaraldehyde solution.
Industrial uses
Polyvinyl alcohol is a tough, whitish polymerthat can be formed into strong films, tubes, andfibers that are highly resistant to hydrocarbonsolvents. Although polyvinyl alcohol is one ofthe few water-soluble polymers, it can be renderedinsoluble in water by drawing or by theuse of cross-linking agents.
Biochem/physiol Actions
Poly(vinyl alcohol) (PVA) is a polyhydroxy polymer, soluble in water. PVA is known to possess high mechanical strength, biocompatibility and non-toxicity. Hence, it serves as a biomedical implant material. Polymerization of vinyl acetate to poly (vinyl acetate), which is then hydrolysed to form PVA. Its application is observed in drug delivery systems, wound dressing, dialysis membranes, artificial skin, surgical repairs and cardiovascular devices.
Pharmacokinetics
Temporarily relieves burning and irritation due to dryness of the eye or from exposure to wind or sun. Lubricates the eyes and helps protect against further eye irritation/dryness .
Safety Profile
Questionable carcinogen with experimental carcinogenic and tumorigenic data by implant route. Flammable when exposed to heat or flame; can react with oxidizing materials. Slight explosion hazard in the form of dust when exposed to flame. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Polyvinyl alcohol is generally considered a nontoxic material. It is
nonirritant to the skin and eyes at concentrations up to 10%;
concentrations up to 7% are used in cosmetics.
Studies in rats have shown that polyvinyl alcohol 5% w/v
aqueous solution injected subcutaneously can cause anemia and
infiltrate various organs and tissues.
(mouse, oral): 14.7 g/kg
(rat, oral): >20 g/kg
Metabolism
Not Available
storage
Polyvinyl alcohol is stable when stored in a tightly sealed container in a cool, dry place. Aqueous solutions are stable in corrosionresistant sealed containers. Preservatives may be added to the solution if extended storage is required. Polyvinyl alcohol undergoes slow degradation at 100°C and rapid degradation at 200°C; it is stable on exposure to light.
Incompatibilities
Polyvinyl alcohol undergoes reactions typical of a compound with secondary hydroxy groups, such as esterification. It decomposes in strong acids, and softens or dissolves in weak acids and alkalis. It is incompatible at high concentration with inorganic salts, especially sulfates and phosphates; precipitation of polyvinyl alcohol 5% w/v can be caused by phosphates. Gelling of polyvinyl alcohol solution may occur if borax is present.
Regulatory Status
Included in the FDA Inactive Ingredients Database (ophthalmic preparations and oral tablets). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of Poly(vinyl alcohol)
| Melting point: | >300 °C |
| Boiling point: | -14.5°C (rough estimate) |
| Density | 1.080 g/cm3 |
| refractive index | 1.3810 (estimate) |
| Flash point: | 79°C |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble (hot) |
| form | Powder |
| color | White to cream |
| PH | 3.5-7.0 (40g/l, H2O, 20℃) |
| Odor | at 100.00?%. odorless |
| Water Solubility | soluble in hot water |
| Merck | 14,7585 |
| Dielectric constant | 1.9(Ambient) |
| Stability: | Stable. Combustible. Dust may form explosive mixtures with air. Incompatible with strong oxidizing agents. |
| IARC | 3 (Vol. 19, Sup 7) 1987 |
| NIST Chemistry Reference | Polyvinyl alcohol(9002-89-5) |
| EPA Substance Registry System | Polyvinyl alcohol (9002-89-5) |
Safety information for Poly(vinyl alcohol)
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H371:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. |
Computed Descriptors for Poly(vinyl alcohol)
Poly(vinyl alcohol) manufacturer
ARRAKIS INDUSTRIES LLP
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran







You may like
-
 PVA-Polyvinyl Alcohol 99%View Details
PVA-Polyvinyl Alcohol 99%View Details -
 Polyvinyl alcohol, Low molecular weight, Hydrolyzed CAS 9002-89-5View Details
Polyvinyl alcohol, Low molecular weight, Hydrolyzed CAS 9002-89-5View Details
9002-89-5 -
 Polyvinyl alcohol, Low molecular weight, Hydrolyzed CAS 9002-89-5View Details
Polyvinyl alcohol, Low molecular weight, Hydrolyzed CAS 9002-89-5View Details
9002-89-5 -
 Polyvinyl alcohol, Medium molecular weight, Hydrolyzed CAS 9002-89-5View Details
Polyvinyl alcohol, Medium molecular weight, Hydrolyzed CAS 9002-89-5View Details
9002-89-5 -
 Polyvinyl alcohol, Medium molecular weight, Hydrolyzed CAS 9002-89-5View Details
Polyvinyl alcohol, Medium molecular weight, Hydrolyzed CAS 9002-89-5View Details
9002-89-5 -
 Polyvinyl alcohol, High molecular weight, Hydrolyzed CAS 9002-89-5View Details
Polyvinyl alcohol, High molecular weight, Hydrolyzed CAS 9002-89-5View Details
9002-89-5 -
 Polyvinyl alcohol, High molecular weight, Hydrolyzed CAS 9002-89-5View Details
Polyvinyl alcohol, High molecular weight, Hydrolyzed CAS 9002-89-5View Details
9002-89-5 -
 Polyvinyl alcohol, Low molecular weight, Hydrolyzed CAS 9002-89-5View Details
Polyvinyl alcohol, Low molecular weight, Hydrolyzed CAS 9002-89-5View Details
9002-89-5
